Response to the NCSE’s Reply to Explore Evolution on Natural Selection
By Casey Luskin, M.S. (Earth Sciences), J.D.
Introduction
In its response to the textbook Explore Evolution: The Arguments For and Against Neo-Darwinism (EE), the National Center for Science Education (NCSE) claims that natural selection can be understood as having served as the primary adaptive force driving “the diversification of life as we know it over the course of several billion years.”1 Despite the NCSE’s bold assurances, scientists have encountered many problems when trying to explain how natural selection acts upon populations to generate complex new biological features. In his Princeton University Press volume Natural Selection in the Wild, biologist John A. Endler writes that “[t]here are six major gaps in our knowledge and understanding of natural selection,”2 namely:
(1) Why does natural selection occur? (2) How does it occur? (3) What kinds of traits are most likely to be affected? (4) What are the effects of simultaneous natural selection of many traits and of the interactions among them? (5) What are the evolutionary dynamics of selected traits? (6) Are genera that are most prone to exhibit natural selection also those that are currently radiating most rapidly?3
Though Endler wrote those words in 1986, debates over natural selection have only intensified among evolutionary biologists. An article in Trends in Ecology and Evolution in 2008 acknowledged the existence of a “healthy debate concerning the sufficiency of neo-Darwinian theory to explain macroevolution.”4 That same year a group of leading evolutionary scientists convened in Altenberg, Austria, to debate the sufficiency of the modern synthesis of evolution to account for biological complexity. Developmental biologist Scott Gilbert was quoted in a Nature article covering the conference stating that “[t]he modern synthesis is good at modeling the survival of the fittest, but not the arrival of the fittest.”5 Biologist Stewart Newman argued in the same article, “You can’t deny the force of selection in genetic evolution … but in my view this is stabilizing and fine-tuning forms that originate due to other processes.”6 Evolutionary paleobiologist Graham Budd was similarly open about deficiencies in neo-Darwinian explanations for key evolutionary transitions, stating: “When the public thinks about evolution, they think about the origin of wings and the invasion of the land … But these are things that evolutionary theory has told us little about.”7
Also in 2008, William Provine, a Cornell University historian of science and evolutionary biologist, gave a talk before the History of Science Society titled “Random Drift and the Evolutionary Synthesis.” An abstract of his talk argues “[e]very assertion of the evolutionary synthesis below is false”:
1. Natural selection was the primary mechanism at every level of the evolutionary process. Natural selection caused genetic adaptation . . . . 4. Evolution of phenotypic characters such as eyes and ears, etc, was a good guide to protein evolution: or, protein evolution was expected to mimic phenotypic evolution. 5. Protein evolution was a good guide to DNA sequence evolution. Even Lewontin and Hubby thought, at first, that understanding protein evolution was the key to understanding DNA evolution. 6. Recombination was far more important than mutation in evolution. 7. Macroevolution was a simple extension of microevolution. 8. Definition of “species” was clear[—]the biological species concept of Dobzhansky and Mayr. 9. Speciation was understood in principle. 10. Evolution is a process of sharing common ancestors back to the origin of life, or in other words, evolution produces a tree of life. 11. Inheritance of acquired characters was impossible in biological organisms. 12. Random genetic drift was a clear concept and invoked constantly whenever population sizes were small, including fossil organisms. 13. The evolutionary synthesis was actually a synthesis.8
The NCSE likes to present the façade of a united front among evolutionary scientists in favor of the basic tenets of neo-Darwinian theory, but other scientists harbor similar doubts about neo-Darwinism. In 2009, Eugene V. Koonin of the National Center for Biotechnology Information stated in Trends in Genetics that due to breakdowns in core neo-Darwinian tenets such the “traditional concept of the tree of life” or the view that “natural selection is the main driving force of evolution” indicate that “the modern synthesis has crumbled, apparently, beyond repair” and “all major tenets of the modern synthesis have been, if not outright overturned, replaced by a new and incomparably more complex vision of the key aspects of evolution.”9 Koonin concludes, “not to mince words, the modern synthesis is gone.”10 Also in 2009, Günter Theißen of the Department of Genetics at Friedrich Schiller University in Jena, Germany, wrote that “[d]espite Darwin’s undeniable merits, explaining how the enormous complexity and diversity of living beings on our planet originated remains one of the greatest challenges of biology.”11 Even more striking criticism of what he called the “dogmatic science”12 of neo-Darwinian thinking can be found in a 2006 paper by Theißen:
Explaining exactly how the great complexity and diversity of life on earth originated is still an enormous scientific challenge. … There is the widespread attitude in the scientific community that, despite some problems in detail, textbook accounts on evolution have essentially solved the problem already. In my view, this is not quite correct.13
Likewise, in a 2007 article in Proceedings of the National Academy of Sciences (PNAS), leading evolutionary biologist Michael Lynch recognized problems with common evolutionary wisdom:
The vast majority of biologists engaged in evolutionary studies interpret virtually every aspect of biodiversity in adaptive terms. This narrow view of evolution has become untenable in light of recent observations from genomic sequencing and population genetic theory. … What is in question is whether natural selection is a necessary or sufficient force to explain the emergence of the genomic and cellular features central to the building of complex organisms.14
In a comment sure to provoke evolutionary biologists who appeal to natural selection as a panacea, Lynch (who is no friend of intelligent design) charges that “simply making the counterclaim that natural selection is all powerful (without any direct evidence) is not much different from invoking an intelligent designer (without any direct evidence).”15
Much like the panselectionists that Lynch complains about, the NCSE’s approach in responding to EE on natural selection is to act as if selection is an all-powerful force that can do any job. The NCSE consistently denigrates attempts to ask deeper questions about the sufficiency of natural selection, asserting it has driven “the diversification of life as we know it over the course of several billion years.” As will be seen in this response to the NCSE, EE’s treatment of natural selection is not only highly accurate, but contains potent arguments against the sufficiency of natural selection. This response will explore the NCSE’s arguments with respect to artificial selection, eye evolution, textbook treatments of natural selection, horse and dog breeding, and the evolution of new genetic information.
I. Even if Artificial Selection Is a Substitute for Natural Selection, It Still Fails
At the beginning of its rebuttal to EE on natural selection, the NCSE states “Explore Evolution claims that Darwin and other biologists use artificial selection as an analogy for the process of natural selection.” If that is what EE says, then as will be shown below, numerous authorities support EE’s contention.
The NCSE’s complaint is that allegedly, “Artificial selection and natural selection are different forms of the same process. Treating the relationship as a mere analogy assumes that differences are greater than they actually are.” The NCSE’s critique apparently misunderstands EE’s arguments. By calling artificial selection an “analogy” to natural selection, EE is not attacking artificial selection as being too different from natural selection to warrant comparison. Rather, EE is arguing something much different, and something very much stronger. EE is arguing that if we grant that, in the NCSE’s words, natural selection and artificial selection are merely “different forms of the same process,” even then the breeding limits encountered during artificial selection indicate that the creative power of natural selection too is limited. EE thus states:
Darwin’s theory states that the unguided force of natural selection is supposed to be able to do what the intelligent breeder can do. But even a process of careful, intentional selection encounters limits that neither time nor the efforts of human breeders can overcome. Consequently, critics argue that by the logic of Darwin’s own analogy, the power of natural selection is also limited. (EE, pg. 91)
By citing intelligent breeders, the NCSE claims that EE uses a “powerful rhetorical opening move” with a “subtle [shift],” again trying to claim that EE is attacking artificial selection as a poor analogy for natural selection because artificial selection employs “intelligent breeder[s].” Yet EE’s argument is that intelligent breeding should make it easier to foster evolutionary change, yet we still encounter limits to evolution. Rather than disqualifying artificial selection from being an analogy for natural selection, artificial selection’s reliance on intelligent breeders demonstrates that even in the best case for evolution, there are still limits to how far populations can evolve. Simply put, the NCSE misunderstands and misrepresents EE’s argument.
Although EE’s argument doesn’t necessarily even challenge the comparison between artificial and natural selection, the NCSE clearly wishes to pretend that there are no differences whatsoever between the two processes. A major obstacle for natural selection that Michael Lynch identifies in his 2007 PNAS article is that in populations of higher organisms, absent strong selection pressure, it is difficult for many features to become fixed. Why? Because selection can easily become overpowered by other forces, such as random genetic drift. Artificial selection, on the other hand, is never subject to these obstacles because the intelligent breeders deliberately select for desired traits and select against undesirable traits. In effect, artificial selection gives desirable traits a selective benefit of 1, and undesirable traits a selective of 0, on a scale from 1 to 0. Nature is far less choosy; selective benefits are usually much less than 1 or even 0.1. In many circumstances, it’s difficult even for beneficial traits to become fixed into a population. In sum, the “population genetics of artificial selection” are more favorable to biological evolution than are the rules of population genetics governing blind and unguided natural selection in the wild.
Other biologists have recognized this point. Loeske E. B. Kruuk states in Philosophical Transactions of the Royal Society of London B that, “in practice components of variance and selection pressures will vary between natural and artificial environments such that studies under artificial conditions have only limited relevance for an understanding of evolution in the wild.”16 Likewise, Montgomery Slatkin and Mark Kirkpatrick call artificial selection a “false analogy” for natural selection because “When artificial selection is applied, the population exposed to it has no choice but to respond.”17 Mary Jane West-Eberhard reframes this argument, observing that there are “abnormally high intensities of artificial selection and consequently high rates of change, due to the fact that the population has ‘no choice but to respond.'”18 EE argues that, even among artificially selected populations, we don’t observe significant biological change.
The NCSE alleges that EE doesn’t allow artificial selection enough time to effect change, because natural selection operates on much longer timescales.19 But West-Eberhard’s reply to Slatkin and Kirkpatrick validates EE’s argument: It is indeed reasonable to expect that the degree of change potentially effected over long periods of time via natural selection could be observed in the shorter timespans covered by artificial selection:
This does not detract from the significance of artificial selection as an assay for genetic variation in natural populations. [Artificial selection] means the potential for a response to selection. Though evolution may not be as rapid in natural populations, the time spans for selection to operate are much longer.20
Thus, West-Eberhard would argue that the change effected through artificial selection can be taken as a representative of potential response to natural selection available in real populations. Once again, EE’s response would be the degree of change observed in artificial selection is limited.
All that is left now is the NCSE’s complaint that EE observes that biologists use artificial selection as an “analogy” for natural selection.
The definition of an “analogy” is “a similarity between like features of two things, on which a comparison may be based: the analogy between the heart and a pump.”21 In other words, analogies are made between two entities that are not identical, but have some similarities and some differences. This seems to describe the exact relationship between artificial selection and natural selection, as recognized by various authorities. In fact, a long tradition of scientists, going back at least to Darwin himself, have compared the two processes, using the word “analogy” and its cognates:
- George John Romane’s famous treatise on evolution from the late 19th century, Darwin and After Darwin, states that “the process of artificial selection is precisely analogous to that of natural selection.”22
- Biologist George St. Clair’s 1873 book Darwinism and Design states, “The experimental argument which lies at the very base of Mr. Darwin’s theory is that man’s process in forming new breeds of pigeons is the analogue of nature’s process in evolving new forms from old—the one is artificial selection, and the other natural selection.”23
- Similarly Charles Clement Coe, in his 1895 Nature Versus Natural Selection: An Essay on Organic Evolution, refers to the “Analogy Between Natural and Artificial Selection.”24
- In more modern times, Mark A. Largent’s chapter in The Cambridge Companion to the “Origin of Species” (co-edited by Michael Ruse) discusses “Darwin’s Analogy between Artificial and Natural Selection in the Origin of Species.”25
- In the 2003 The Cambridge Companion to Darwin, C. Kenneth Waters notes that, “Darwin argued for the adequacy of natural selection by appealing to the analogy between artificial and natural selection.”26
- A chapter in the National Academy Press volume In the Light of Evolution: Adaptation and Complex Design observes that “In the opening chapter of the Origin of Species, Charles Darwin introduced the idea of natural selection with an analogy to domestication.”27
- M.J.S. Hodge observes in the 1992 Harvard University Press book Keywords in Evolutionary Biology that Darwin coined the term “natural selection” as an analogy for artificial selection: “To understand the history of the term ‘natural selection’ both before and after this moment in the Origin, we have, therefore, to look not for a sequence of explicit definitional equations but, rather, for the reasons why people, starting with Darwin himself, have felt themselves able to grasp and wield the concept adequately in the absence of consistent, authoritative definitional analyses of the term. In Darwin’s own case, the term itself was a secondary matter; what really counted was his argument for the analogy that the term was coined to signify, the analogy between man’s selection and nature’s.“28
Just like EE, a great many authorities call artificial selection an “analogy” for natural selection. Not only that, but like all analogies, this one is imperfect—a point the NCSE would ignore, since the imperfection of the analogy counts against the creative power of natural selection, not for it.
Apparently finding little else to complain about, the NCSE also attacks EE for spending too much time discussing the domestic breeding of sheep, stating: “There is, in fact, a surprising amount of discussion about sheep and an unusual abundance of sheep photographs in this chapter (the reader may choose to complete this straight-line with an appropriate remark of his or her choosing).” No response to the NCSE’s juvenile comment is necessary.
II. An Eye For Trouble
Using a similar tone, the NCSE states:
Explore Evolution asks, “But can this three-step process construct organs as complex as an eye?” This hackneyed canard about the alleged irreducible complexity of the eye rises again.
Perhaps this “hackneyed canard” keeps coming up because standard evolutionary explanations of the eye are woefully inadequate. As James Shapiro writes:
One of the most important questions in evolution is: How can new adaptations originate? This is a difficult question, because most evolutionary novelties, such as the eye or the wing, involve the orchestrated expression of many different loci, a number of which act in the expression of multiple phenotypes. Conventional explanations that randomly generated advantageous changes in complex characters accumulate one locus at a time are unconvincing on both functional and probabilistic grounds, because there is too much interconnectivity and too many degrees of mutational freedom.29
Classical explanations for the evolution of the eye assume that the eye can be built via such small, step-by-step changes. Darwin believed the eye could evolve under a scheme of “fine gradations,” but standard evolutionary accounts for the origin of the eye fall far short of that standard: they lack details, ignore biochemical complexity, and in fact invoke sudden and abrupt appearance of key components of eye morphology.
For example, all accounts of eye evolution start with a fully functional eyespot. As Mark Ridley’s textbook Evolution explains, one commonly cited model of eye evolution…
began with a crude light-sensitive organ consisting of a layer of light-sensitive cells sandwiched between a darkened layer of cells and a transparent protective layer above. The simulation, therefore, does not cover the complete evolution of an eye. To begin with, it takes light sensitive cells as given … and at the other end it ignores the evolution of advanced perceptual skills (which are more a problem in the evolution of the brain than the eye).30
Ridley calls it “not absurd”31 to assume simple light sensitive cells as a starting point, but evolutionary biologist Sean B. Carroll cautions that we should “not be fooled by these eyes’ simple construction and appearance. They are built with and use many of the ingredients used in fancier eyes.”32 Likewise, after reviewing some of the basic biochemistry underlying the processes that allow vision, Michael Behe (responding to Richard Dawkins) observes: “Remember that the ‘light-sensitive spot’ that Dawkins takes as his starting point requires a cascade of factors, including 11-cis retinal and rhodopsin, to function. Dawkins doesn’t mention them.”33 In fact, no accounts of the evolution of the eye provide an explanation for this always-assumed starting point.
In addition to assuming the abrupt appearance of a functional eyespot, standard accounts of the evolution of the eye invoke the abrupt appearance of key features of advanced eyes such as the lens, cornea, and iris.34 Of course the development of each of these features whole and functional would undoubtedly increase visual acuity, but where did these parts suddenly come from in the first place? As Scott Gilbert put it, such evolutionary accounts are “good at modeling the survival of the fittest, but not the arrival of the fittest.”35
As an example of a hyper-simplistic account of eye evolution, Francisco Ayala’s Darwin’s Gift asserts that, “Further steps—the deposition of pigment around the spot, configuration of cells into a cuplike shape, thickening of the epidermis leading to the development of a lens, development of muscles to move the eyes and nerves to transmit optical signals to the brain—gradually led to the highly developed eyes of vertebrates and cephalopods (octopuses and squids) and to the compound eyes of insects.”36 Ayala’s explanation is vague, and shows no appreciation for the biochemical complexity of these visual organs.
Thus, regarding the configuration of cells into a cuplike shape, Michael Behe asks (while responding to Richard Dawkins on the same point):
And where did the “little cup” come from? A ball of cells—from which the cup must be made—will tend to be rounded unless held in the correct shape by molecular supports. In fact, there are dozens of complex proteins involved in maintaining cell shape, and dozens more that control extracellular structure; in their absence, cells take on the shape of so many soap bubbles. Do these structures represent single-step mutations? Dawkins did not tell us how the apparently simple “cup” shape came to be.37
Likewise, mathematician David Berlinski has assessed the alleged “intermediates” for the evolution of the eye and observes that the transmission of data signals from the eye to a central nervous system for data processing, which can then output some behavioral response, comprises an integrated system that is not amenable to stepwise evolution:
Light strikes the eye in the form of photons, but the optic nerve conveys electrical impulses to the brain. Acting as a sophisticated transducer, the eye must mediate between two different physical signals. The retinal cells that figure in Dawkins’ account are connected to horizontal cells; these shuttle information laterally between photoreceptors in order to smooth the visual signal. Amacrine cells act to filter the signal. Bipolar cells convey visual information further to ganglion cells, which in turn conduct information to the optic nerve. The system gives every indication of being tightly integrated, its parts mutually dependent.
The very problem that Darwin’s theory was designed to evade now reappears. Like vibrations passing through a spider’s web, changes to any part of the eye, if they are to improve vision, must bring about changes throughout the optical system. Without a correlative increase in the size and complexity of the optic nerve, an increase in the number of photoreceptive membranes can have no effect. A change in the optic nerve must in turn induce corresponding neurological changes in the brain. If these changes come about simultaneously, it makes no sense to talk of a gradual ascent of Mount Improbable. If they do not come about simultaneously, it is not clear why they should come about at all.
The same problem reappears at the level of biochemistry. Dawkins has framed his discussion in terms of gross anatomy. Each anatomical change that he describes requires a number of coordinate biochemical steps. “[T]he anatomical steps and structures that Darwin thought were so simple,” the biochemist Mike Behe remarks in a provocative new book (Darwin’s Black Box), “actually involve staggeringly complicated biochemical processes.” A number of separate biochemical events are required simply to begin the process of curving a layer of proteins to form a lens. What initiates the sequence? How is it coordinated? And how controlled? On these absolutely fundamental matters, Dawkins has nothing whatsoever to say.38
Nor does the NCSE, apparently, other than declaring that to ask hard questions about evolution is merely to raise a “hackneyed canard.” In sum, standard accounts of eye evolution fail to explain the evolution of key eye features like:
- The biochemical evolution of the fundamental ability to sense light
- The origin of the first “light sensitive spot”
- The origin of neurological pathways to transmit the optical signal to a brain
- The origin of a behavioral response to allow the sensing of light to give some behavioral advantage to the organism
- The origin of the lens, cornea and iris in vertebrates
- The origin of the compound eye in arthropods
At most, accounts of the evolution of the eye provide a stepwise explanation of “fine gradations” for the origin of more or less one single feature: the increased concavity of eye shape. The NCSE’s writer undoubtedly finds it satisfying to dismiss these other problems, but meanwhile, compelling explanations for the evolution of the eye are lacking and curious scientists find these questions are still worth asking.
III. The NCSE’s Double Standards on EE and Other Biology Texts
When unable to find legitimate fault with EE on natural selection, the NCSE resorts to stretches and distortions, claiming: “Explore Evolution believes that the only examples of natural selection in biology textbooks are Darwin’s finches and Kettlewell’s peppered moth experiments.” EE says nothing of the kind. What EE actually states is that “Biology textbooks cite two classic examples to support the claim that natural selection can produce small-scale change over a short time” (EE, pg. 88). It then discusses the Galápagos finches and peppered moths. Nowhere does EE claim that these are the only two examples given in all biology textbooks.
Oddly, the NCSE then cites from Campbell’s Biology (6th Ed.) regarding none other than the Galápagos finches, and later admits that Raven and Johnson’s biology textbook covers these examples, validating EE’s point that these examples are common in textbooks. In fact, many more textbooks could be cited to show just how common the peppered moths and Galápagos finches are in textbooks as examples of natural selection:
Peppered moths:
- Douglas Futuyma, Evolution (Sinauer, 2005)
- Holt’s Life Science (Holt, Rinehart, and Winston, 2001)
- Sylva S. Mader, Essentials of Biology (McGraw Hill, 2007)
- Strauss and Lisowski, Biology: The Web of Life (Addison-Wesley, 2000)
- Glencoe’s Biology: The Dynamics of Life (Florida Edition, 2006)
- Sylvia S. Mader, Biology (10th ed., McGraw Hill, 2007)
- Scott Freeman, Biological Science (3rd ed., 2008)
Galápagos finches:
- Kenneth Miller and Joseph Levine, Biology (Pearson/Prentice Hall, 2008)
- David Savada, H. Craig Heller, Gordon H. Orians, William K. Purves, David M. Hillis, Life: The Science of Biology (8th ed., Sinauer, W. H. Freeman, 2008)
- Douglas Futuyma, Evolution (Sinauer, 2005)
- Holt’s Life Science (Holt, Rinehart, and Winston, 2001)
- Sylva S. Mader, Essentials of Biology (McGraw Hill, 2007)
- Strauss and Lisowski, Biology: The Web of Life (Addison-Wesley, 2000)
- Glencoe’s Biology: The Dynamics of Life (Florida Edition, 2006)
- Modern Biology (Holt Rinehart and Winston, 2002)
- Raver’s Biology: Patterns and Processes of Life (J. M. Lebel, 2004)
- Collen Belk & Virginia Borden Maier, Biology: Science for Life (Pearson / Benjamin Cummings, 2010)
- Sylvia S. Mader, Biology (10th ed., McGraw Hill, 2007)
- Glencoe’s Biology: An Everyday Experience (Glencoe, 2003)
- Scott Freeman, Biological Science (3rd ed., 2008)
Clearly, peppered moths and Galápagos finches are two of the most common examples of natural selection used in modern biology textbooks, and Explore Evolution is justified in focusing on them and stating that “Biology textbooks cite two classic examples” to support natural selection.
The NCSE further complains that “[n]either HIV [anti-viral drug resistance] nor insecticide [resistance] even rate a mention in the index of Explore Evolution.” There’s nothing wrong with textbooks mentioning these examples, but the NCSE holds EE to a double standard as a number of standard introductory biology textbooks do not mention HIV anti-viral drug resistance39 or insect resistance to insecticides40 as examples of natural selection.
In a string of contrived complaints, the NCSE attacks EE for its discussion of sickle cell anemia as “purely … an example of a mutation” and not natural selection. But then the NCSE effectively concedes this complaint is invalid by admitting that EE “mentions that sickle cell anemia can be beneficial under some circumstances,” and is thus favored by natural selection. As EE states, the sickle cell anemia “mutation can be beneficial to some people, giving them a measure of protection against malaria … In this way, a mutation that usually leads to an early death is actually beneficial, giving protection against malaria.” (EE, pg. 99)
The NCSE then complains that EE allegedly doesn’t “employ a truly inquiry-based approach by inviting students to develop and test hypotheses about malarial resistance.”
There would be nothing wrong with such a textbook exercise, but the NCSE’s complaint exposes the type of “inquiry” expected by the NCSE. The NCSE would apparently wish all “inquiry” activities to explore competing hypotheses about neo-Darwinian evolution rather than exploring more fundamental questions about the overall sufficiency of neo-Darwinism as an explanatory paradigm. As was seen in the eye example in part II, the NCSE doesn’t want inquiry that investigates questions that are too deep and fundamental about the sufficiency of neo-Darwinian mechanisms.
The NCSE’s final attempt at a knock-out argument against EE’s examples of natural selection is that one particular full-length upper division college-level evolutionary biology textbook—Douglas Futuyma’s Evolutionary Biology—covers some topics not in EE, such as latitudinal gradients in allele frequency in fruit fly populations, guppy coloration changes in response to local predation pressures, and paleontological evidence of snail shell shapes showing stabilizing selection. Is it even remotely reasonable to expect EE to cover many of the esoteric topics found in upper-division college level evolutionary biology textbooks?
Futuyma’s examples are fascinating to be sure, but EE is designed for use in an introductory biology course, not an upper division university course in evolutionary biology. To demonstrate the NCSE’s unreasonable double-standards, not a single introductory-level college biology textbook we surveyed covered all of these alleged examples of natural selection, and most of them covered none of these particular examples.41
Apparently the NCSE can only find fault with EE when applying unreasonable double standards. At this point, one must look past the double-standards and nitpicking and ask the deeper question: even if EE discussed all of these examples, what would that show?
The examples cited by the NCSE—latitudinal gradients in allele frequency in fruit fly populations, guppy coloration changes in response to local predation pressures, paleontological evidence of snail shell shapes showing stabilizing selection, HIV anti-viral drug resistance, and insecticide resistance in insects—just like peppered moths and the Galápagos finches, all show only small-scale changes within populations. For all its hard work digging through biology textbooks, the NCSE has, in the end, only validated EE’s point: “Far from proving an ability to produce extraordinary change, each of the previously mentioned textbook examples actually illustrates the opposite: that natural selection’s capacity to produce change is limited.” (EE, pg. 92)
IV. Horses and Dogs
A. The NCSE’s False Dichotomies and Improper Use of the “Two Model” Approach
The NCSE claims that EE’s discussion of limits to change in artificial selection is “essentially a restatement of the creationist doctrine that types—or baramins—cannot evolve into one another.” The NCSE here employs a false dichotomy, arguing as if anyone who challenges neo-Darwinian evolution is necessarily arguing for some creationist position, as if these were the only two possible positions that can be held.
In fact, arguments against evolution do not necessarily entail arguments for creation science. The NCSE knows this, yet its argument impliedly adopt the long-falsified “two model” approach of creation scientists by confusing EE’s arguments for limits to biological change with arguments for creationism. EE does not argue for creationism, and of course the NCSE does not believe in the “two model” approach, showing that the NCSE is cynically misrepresenting EE by claiming the textbook argues for creationism.
B. The NCSE Misstates EE’s Arguments on Artificial Selection
The NCSE further attempts to critique EE as follows:
Explore Evolution argues that if natural selection cannot produce a certain change in a matter of decades, it could never produce that change. This is nonsensical on its face, and does not accurately reflect basic knowledge about natural selection and population genetics stretching back to the 1920s. It also misstates the effects that animal husbandry has been shown to have on domestic species.
The NCSE again engages in misrepresentation. In this section, the NCSE should be rebutting EE’s arguments about artificial selection, not natural selection. As noted above, artificial selection is able to dramatically speed up the rate at which change takes place by deliberately selecting for certain traits. Thus, we can expect that what takes many thousands of years for natural selection to accomplish might be happen much faster by artificial selection.
The NCSE implies that horse and dog breeding have only been going on for “decades,” yet domestication of animals like horses and dogs began many millennia ago. It is estimated that dog domestication began as early as 30,000 years ago, and horse breeding goes back perhaps as far as 5,000 years.42 Indeed, EE notes that dogs have experienced “thousands of years of selective breeding.” (EE, pg. 90) Given that natural speciation events are said to take place in as little as a few hundred generations, or about 5,000 years,43 and given that artificial selection only speeds up the process of change, it can be assumed that we should be able to witness dramatic biological change in these cases.
Additionally, the NCSE’s argument illegitimately shifts the burden of proof. The NCSE (and many pro-evolution biology textbooks) ask students to accept that natural selection can effect great changes in species based upon evidence that only shows small changes within species across centuries or a few millennia (not merely “decades”). The NCSE calls EE’s arguments “nonsensical,” but it is the neo-Darwinian evolutionists who have the burden of providing evidence to justify their extrapolation from the changes effected by artificial selection to grand macroevolutionary changes. Since we have to work with the data that is available, EE is fully justified in observing that animal breeders have reached limits.
C. The NCSE Bets on the Wrong Horse
The NCSE claims that when EE states, “Horse breeders have not significantly increased the running speed of thoroughbreds, despite more than 70 years of trying” (p. 90), that the textbook is “inaccurate on at least one count, and … misrepresents the source they [sic] cite.”
The NCSE first concedes that EE’s source—a Nature article authored by credible authorities—makes the argument for which EE cites it, stating, “Gaffney and Cunningham (1988), the paper they cite to justify the sentence, do find that winning race times have not changed.” So what’s the problem? The NCSE then wrongly claims that Gaffney and Cunningham’s argument contradicts EE’s argument:
[Gaffney and Cunningham] end the paper stating, “We conclude that the explanation for the lack of progress in winning times is not due to a lack of genetic gain in the thoroughbred population as a whole.” Genetic gain in the population as a result of selective breeding is the very definition of artificial selection.
Gaffney and Cunningham’s 1988 paper in Nature certainly does make that argument, but a closer examination of their paper justifies what EE says and shows that the NCSE’s argument is highly misleading. Gaffney and Cunningham contradict nothing in EE.
Gaffney and Cunningham’s paper shows that the speed of the fastest horses is not increasing, but they note that their analysis of race times “refer[s] to the population as a whole, whereas the trend in winning times relates only to the best horses in the best races.”44 Thus, their analysis shows that the fastest horse times haven’t changed, showing an upper genetic limit. This supports EE’s central argument about limits to racehorse speed.
So what is changing? Gaffney and Cunningham show that the population of racehorses as a whole is getting faster, but that is simply because the population is more closely approximating an observed speed limit of the fastest horses. In other words, the genes for the fastest horses seem to be becoming more and more common in the racehorse population, so more and more of the population is beginning to achieve a maximum genetic potential for speed. But the maximum genetic potential has not changed. EE is concerned with the maximum genetic potential, and thus this paper does not challenge EE’s central argument that there are limits to the speed of racehorses.
Gaffney and Cunningham’s findings make sense, and can be thought of like this: Many decades ago, breeders were occasionally able to breed for a horse that more-or-less maxed out the genetic potential for speed within the population of racehorses. Over time, good breeding practices have allowed such prized genes to become more and more common in the racehorse population, such that the average horse in the population is getting faster.
But this does not imply that the fastest horses are getting faster, or that any new limit of maximum genetic potential has been breached. All it means is that the genes for the fastest horses are becoming more common.
Gaffney and Cunningham’s results indicate that a genetically imposed speed limit for racehorses DOES exist. The fact that more and more racehorses are coming to approximate that limit does not disprove the existence of the limit. Since EE’s point is that there are limits to the amount of biological change that can be effected by artificial selection, it seems that Gaffney and Cunningham’s paper indeed confirms EE’s central point.
Continuing its misguided train of thought, the NCSE then quotes a later paper by Ernst Bailey (1998) in Genetics stating:
…breeders and horse-racing enthusiasts state they pay little attention to winning times. Instead, riders, horse owners, breeders, and bettors are rewarded for horses that win races, regardless of time, and little effort is made to “beat the clock.”45
First, the NCSE must be counting on its readers not digging up its sources, because Bailey’s prior paragraph summarizes Cunningham and Gaffney’s 1988 Nature paper as concluding exactly what EE cites the paper as saying. Bailey thus writes:
But at the same time these same investigators reported that winning times have not improved significantly during the last 50 years for ”classic races,” for example, races designed to match the best horses each year. Cunningham noted that winning times had been especially static for distance races and suggested that a physiological limit might have been reached, for example, for dealing with lactic acid buildup in muscle during performance. Therefore, although horses exhibited genetic variation for racing performance and the population continued to exhibit genetic gain during the period of study, the best times did not improve.46
The NCSE doesn’t quote this section, but in fact this is exactly how EE summarizes Cunningham and Gaffney.
Second, the portion of Bailey’s article that the NCSE does quote simply notes that absolute time is unimportant to breeders. However, this is irrelevant to the question of whether breeders are bumping up against genetic limits to the maximum speed of racehorses. If relative time is the main concern, breeders still want to have the faster horse. Thus, breeders most certainly do aim to produce faster horses with the best genes for running fast, and this should, over time, lead to faster maximum horse speeds if that is genetically possible. Horse enthusiasts may not care if their favorite racehorse can “beat the clock,” but they still want to “beat all the other horses,” so there’s always a drive to produce the faster horses. If there is increased maximum speed to be gained by artificial selection, we should see it, because every breeder aims to breed horses faster than the next horse.Thus, after saying “it is not the case that horse breeders have tried to increase the absolute time in which their horses complete races, but to ensure that their horses run faster than the other horses in a given race,” the NCSE’s next statement is highly illogical:
It is therefore impossible to know whether contemporary horses would run faster than famous racehorses like Seabiscuit or Secretariat if they ran against one another, or whether contemporary horses as a whole are faster in absolute terms than horses were 70 years ago.
The NCSE is wrong. Again, it is not necessary for breeders to be thinking of “absolute times” in order for them to be breeding for faster horses; breeding for the fastest relative time will likewise lead to faster maximum speeds, if such speeds are attainable.
Indeed, Bailey goes on to suggest potential limits to racehorse performance:
In this regard, Jim Rooney (pers. comm., this conference), an expert on biomechanics of the horse, noted that if there is a limit on performance of the racehorse, it may be on the ability of the horse to remain sound in the face of the tremendous stresses of racing.47
Finally, the NCSE closes its section on racehorses by citing Bailey to document today’s supposedly “slower” racetracks: “Furthermore, ‘fast tracks’ are notoriously bad for the health of horses, causing damage to bones and tendons. Consequently, track surfaces are often treated to be softer, slower, and less likely to cause stress on the horse. Thus, modern racetracks may be slower than the tracks of 50 years ago.”48 Bailey’s argument is non-rigorous and purely anecdotal. He cites no evidence to document the effects of any tracks on race times. It is a convenient strategic ploy for the NCSE to put forth an argument that is basically impossible to evaluate.
More importantly, not all authorities agree with Bailey. For example, successful thoroughbred horse breeder Paul H. Rothfuss49 writes that “Compared with decades ago, the dirt race track surfaces of today are lightning-fast.”50
After writing this section, I contacted Mr. Rothfuss, an expert in the field of thoroughbred racehorses, and asked his opinion of whether racetracks today are being made “slower,” than years past. He wrote in reply:
[Bailey] then wrote: “Consequently, track surfaces are often treated to be softer, slower, and (thus) less likely to cause stress on the horse.” If surfaces are slower and there is less stress on the horses, why are there (allegedly) more breakdowns today than in the past?
Also, [Bailey’s] thought is a disconnect with the sentence that preceded it. It presumes that race track superintendents, realizing that their fast surfaces may have been causing injuries, reacted to that realization by “slowing” the surfaces down. I want to see his sources for this “thought” that he presents as fact. Obviously, I disagree with the thought and I believe that ninety-nine percent of the trainers would agree with me.
The reality is quite the opposite. In the mistaken belief that faster times make more people come to see Thoroughbreds run, the track supers are constantly grooming their surfaces in an effort to produce that “best” surface that allows the horse to run faster than ever before. They are NOT intentionally trying to cause breakdowns. They ARE intentionally trying to make their surfaces both faster and yes, safer. But I don’t think there’s a track super in the USA who intentionally makes his surface “slow.”
[Bailey] concluded with: “Thus, modern racetracks may be slower than the tracks of 50 years ago.” He conveniently used the word “may” which, I suppose, gets him off the hook. Remove that word and this statement simply cannot be true because the “science” of maintaining a race track today is so much better than it was 50 years ago. So are the materials used in the cushion and so is the equipment that is used in the maintenance. I 100 % disagree and would require that he cite “chapter and verse” and prove his conclusion with verifiable, expert evidence.
In the 1950s and ’60s, any Thoroughbred (racing anywhere other than California) who could run three-quarters of a mile (six furlongs) in less than 1:12 was considered a pretty good horse, and one who could go in ten and change, or faster, was considered to be very fast. Today, horses routinely go in 1:10 and change and nobody blinks an eye, and they run in 1:08 in CA, even on the artificial surfaces.
“Slower” surfaces today? Not on your life!“51
Rothfuss thus attributes faster race times to faster racetracks. In short, regarding the existence of genetic limits to maximum racehorse speed, NCSE has not made a dent in EE’s arguments.
D. It’s a Dog Eat Dog World
The NCSE opens its response to EE on artificial selection of dog breeds with its typical disparaging rhetorical style, stating: “The book’s dismissal of variation within dogs is, if possible, even more disingenuous” than EE’s treatment of horses. Yet as we’ve just discussed, EE’s treatment of horses was accurate and supported by credible authorities, while the NCSE’s rebuttal was mistaken.
Regarding the NCSE’s commentary on dog breeding, the NCSE is to be commended for its elaboration of the different sizes and shapes of dog limbs and skulls. However EE contests none of this. In fact EE happily acknowledges the existence of “an array of dog breeds with strikingly different sizes and shapes.” (EE, pg. 90, emphasis added)
The diversity of dog limb and skull morphology is not news to anyone, and contradicts nothing in EE. However, as EE states, “No one has ever bred a dog lighter than a few pounds, or heavier than about 150 pounds, despite thousands of years of selective breeding. Critics say that the experimental evidence reveals definite, discoverable limits on what artificial selection can do.” (EE, pg. 90) Additionally, EE observes the many health problems encountered by certain dog breeds, whose unfit morphologies would be unlikely to persist in the wild. This implies that breeders, again, are hitting limits.
In contrast, the NCSE argues based upon variation in dog limb and skull morphology that, “There is no evidence in these data to suggest that dogs have reached any inherent limits to their evolution or to the powers of natural selection.” But given that, as EE observes, we are hitting limits and many breeds face health problems, it seems that the NCSE is wrong.
Additionally, evolutionary authorities themselves might well disagree with the NCSE’s resistance to accepting the existence of limits on dog breeding. Austin Hughes explains in the journal Heredity that limits encountered during artificial selection and selective breeding demonstrate there is not always “abundant genetic variation on which to act”:
The following are three major areas of misconception among the Neo-Darwinists…
Artificial selection on quantitative traits was taken as a model of the evolutionary process. It was easily shown, in agriculture or in the laboratory, that populations of most organisms contain sufficient additive genetic variance to obtain a response to selection on quantitative traits, such as measures of body size or increased yield of agriculturally valuable products such as milk in dairy cattle or grain size in food plants.
Generalizing from this experience, it was assumed that natural populations are endowed with essentially unlimited additive genetic variance, implying that any sort of selection imposed by environmental changes will encounter abundant genetic variation on which to act. Moreover, this model was extended to evolutionary time as well as ecological time. This way of thinking ignored the substantial evidence from selection experiments that the response to selection on any trait essentially comes to a halt after a number of generations as the genetic variance for the trait in question is depleted; thereafter, further progress depends on the introduction of new variants either through outcrossing or new mutations (Falconer, 1981).52
Likewise, Ernst Mayr cautions against overstating the creative power of natural selection:
Some enthusiasts have claimed that natural selection can do anything. This is not true. Even though “natural selection is daily and hourly scrutinizing, throughout the world, every variation even the slightest,” as Darwin (1859:84) has stated, it is nevertheless evident that there are definite limits to the effectiveness of selection.53
Mayr goes on to cite “[t]he limited potential of the genotype” which shows “severe limits to further evolution”54:
The existing genetic organization of an animal or plant sets severe limits to its further evolution. As Weismann expressed it, no bird can ever evolve into a mammal, nor a beetle into a butterfly. Amphibians have been unable to develop a lineage that is successful in salt water. We marvel at the fact that mammals have been able to develop flight (bats) and aquatic adaptation (whales and seals), but there are many other ecological niches that mammals have been unable to occupy. There are, for instance, severe limits on size, and no amount of selection has allowed mammals to become smaller than a pygmy shrew and the bumblebee bat, or allow flying birds to grow beyond a limiting weight.55
EE would go even further than Mayr and suggest that if there are undeniable evolutionary limits on traits like size or the ability of certain clades to occupy certain niches, then this calls into doubt many evolutionary claims, such as Mayr’s confident assertion that ground dwelling mammals evolved into bats and whales. Nonetheless, Mayr has conceded important points, validating that some of the very types of limits to evolution discussed by EE can and do exist.
V. More Limits to Evolution
Many neo-Darwinian evolutionists have argued that macroevolution is simply repeated rounds of microevolution, and that given enough time microevolutionary changes can add up to extremely grand macroevolutionary changes. As Sean B. Carroll observes, “Many geneticists assert that macroevolution is the product of microevolution writ large.”56
Similarly, in his textbook Evolution, Mark Ridley contends, “In some cases, macroevolution will likely be extrapolatable from microevolution.”57 David Sepkoski writes that “many biologists argue that, insofar as broad macroevolutionary trends can be extrapolated from microevolutionary processes, everything needed to explain evolution can be found in Darwin’s theory.”58 Joseph Travis and David N. Resnick likewise observe that Darwin’s major accomplishment was to unify the causes of both microevolution and macroevolution:
The most revolutionary feature of Darwin’s On the Origin of Species (1859) was to propose natural selection as the single unifying mechanism that causes both micro- and macroevolution. Darwin argued that macroevolution is just microevolution writ large, or that the process we see and study as the cause of microevolution will, given sufficient time, also cause everything that we attribute to macroevolution. He argued that natural selection, which causes the evolution of the adaptations discussed throughout this essay is also responsible for the origin of all levels of biological complexity and for the origin of biological diversity, or all the species that have been found on the earth throughout its history.59
Textbooks also make such extrapolations. Peter H. Raven & George B. Johnson’s Biology (6th ed. 2002) asks: “Is microevolution (evolution within a species) the mechanism that has produced macroevolution (evolution among species)? Most biologists that have studied the problem think so.”60 Likewise, Campbell’s Biology makes a clear extrapolation from microevolution to macroevolution, stating: “The cumulative change during millions of speciation episodes over vast tracts of time must account for macroevolution, the level of change that is evident over the time scale of the fossil record.”61
Keeping these points in mind, let’s assess some of the NCSE’s arguments on limits to evolution.
A. Tall Neo-Darwinian Extrapolations
As we have just seen, biologists commonly extrapolate from the small-scale processes of microevolution to much larger-scale processes of macroevolution. On page 92, EE offers an analogy to explain such neo-Darwinian extrapolations. The analogy is that neo-Darwinists might contend that if a tree could grow from 1′ to 5′ in 5 years, then in 1000 years they might expect a tree might grow to a height of 1000′. Fundamentally, EE’s mode of reasoning in this analogy does not misrepresent the common and simplistic extrapolation-based arguments of many neo-Darwinists.
The NCSE complains the analogy is “ridiculous” because trees do not grow to 1000 feet tall. But EE could have used an example of a tree growing to 100′ in 100 years, and it’s likely that the NCSE would not complain because such an extrapolation sounds reasonable.
Thus, neo-Darwinists certainly do make these kinds of simplistic extrapolations and the NCSE’s complaint is over the degree of extrapolation, not the kind; trees do not grow to 1000′ but these sorts of simplistic extrapolations are commonly made under neo-Darwinian thinking.
The NCSE might feel that the tree analogy goes too far, but that’s exactly the point made by critics of neo-Darwinism: not all extrapolations are appropriate, and sometimes neo-Darwinian extrapolations go too far.
The appropriateness of this 1000′ tree analogy thus depends on your perspective: skeptics of neo-Darwinism find it entirely appropriate because they believe that neo-Darwinists make unwarranted extrapolations; die-hard defenders of neo-Darwinism find the analogy inappropriate because they feel their extrapolations are reasonable.
The NCSE might protest the use of the 1000′ tree analogy, but EE is justified if neo-Darwinists do indeed make over-extrapolations. What is ironic is that the NCSE makes something very much like this type of extreme yet simplistic extrapolation argument in its response to EE on natural selection. The NCSE states:
What these data show is that dog breeders have already managed to produce animals which break new morphological ground. Whatever limits might seem to exist if we look at the shapes and sizes of wild canids have been surpassed by the work of dog breeders.
Whatever limits natural selection has, they have prevented the evolution of variation beyond that seen within the rest of the entire order Carnivora (dogs, cats, bears, foxes, weasels, etc.), all within the last few thousand years. Natural selection may well have limits, but if the limits are that loose, they would not prevent the diversification of life as we know it over the course of several billion years.
So according to the NCSE, if dog breeders can change skull and limb morphologies in dogs over a few thousand years of dog breeding, then we can extrapolate to conclude that natural selection can produce “the diversification of life as we know it over the course of several billion years.” Obviously, producing all the diversity of life requires much more than changing the dimensions of limb and cranial bones in dogs: it must involve the origin of limb and cranial bones to begin with, the origin of the hundreds of cell types found in organisms with limbs and crania, not to mention the origin of thousands of proteins and protein machines built in cellular factories inside those cells, as well as the origin of innumerable other features including the most complicated machine in the universe—the mammalian brain—to fill the cranium. The NCSE calls the 1000′ tree analogy “ridiculous,” but perhaps that charge tells us more about the weaknesses in the NCSE’s own arguments than it does about any fault of EE.
In fact, it might have been more appropriate for EE to demand a 1,000,000,000,000′ tree.
Tree analogies aside, EE’s main point here is that neo-Darwinists extrapolate from microevolution to macroevolution. EE uses the tree analogy to bring out the extrapolations of the many evolutionists who, continuing in Darwin’s tradition, argue that, in Travis and Resnick’s words, “macroevolution is just microevolution writ large, or that the process we see and study as the cause of microevolution will, given sufficient time, also cause everything that we attribute to macroevolution.” EE’s point is completely valid, since examples of neo-Darwinists making comparable extrapolations from microevolution to macroevolution are not hard to find. It seems entirely reasonable and responsible for EE to encourage caution when making extrapolations from one process to another, because not all extrapolations are warranted.
Rather than complaining about the analogy given in EE for teaching purposes, the NCSE would do better to focus on justifying such grand extrapolations. As a 2009 paper in BioEssays put it, “Elucidating the materialistic basis of the Cambrian explosion has become more elusive, not less, the more we know about the event itself, and cannot be explained away by coupling extinction of intermediates with long stretches of geologic time, despite the contrary claims of some modern neo-Darwinists.”62 Darwin-skeptics are not unjustified in finding that neo-Darwinian extrapolations from microevolution to macroevolution are lacking.
B. The NCSE’s Free Association Arguments about Young Earth Creationism
Lacking a good rebuttal to the tree-growth analogy, the NCSE apparently feels so desparate that it decides to stoop to implying that EE is making an argument for young earth creationism. But since there is no argument for young earth creationism in EE, the NCSE simply starts talking about young earth creationism (YEC) in hopes that the reader will make some kind of mental connection based on free-association reasoning:
This claim is related to the “young earth” creationist belief that the earth is only a few thousand years old. In this belief system, there has not been enough time for speciation to occur, given the rate of change that we can observe in most populations. So it is necessary for them to deny reality (observations of speciation) in order to validate a creationist perspective on the age of the earth. An age, by the way, that is about 0.00000002% of the approximately 3 billion years over which biological evolution has proceeded.
The NCSE hopes that if it talks about problems with YEC, the reader will actually believe that EE is promoting YEC. The reality is that there is nothing in EE, whether in its chapter on natural selection or anywhere else, that argues for or implies a young earth. EE plainly observes that trilobite fossils are found in “in rock layers covering a period of about 300 million years.” (EE, pgs. 16-17) Regarding the Cambrian explosion, the textbook observes that “about 530 million years ago, more than half of the major animal groups (called phyla) appear suddenly in the fossil record.” (EE, pg. 22) In fact, page 18 of EE is a full-page diagram of the entire geological timescale, with all of the standard geological ages included, and of course no criticism of the timescale whatsoever.
C. The NCSE’s Citation Bluffs and The Information Problem
Not long before the 2005 Kitzmiller v. Dover trial began, then-National Center for Science Education staff member Nicholas Matzke claimed to a reporter that “The origin of genetic information is thoroughly understood.”63 During the Dover trial, plaintiffs’ expert witness, biologist Kenneth Miller, testified that he presented Judge John E. Jones with “more than three dozen scientific studies showing the origin of new genetic information by these evolutionary processes.”64 The plaintiffs’ attorneys, working with the NCSE, successfully convinced Judge Jones to parrot Miller by stating in the Kitzmiller v. Dover ruling that Miller had “pointed to more than three dozen peer-reviewed scientific publications showing the origin of new genetic information by evolutionary processes.”65
Virtually all of those “publications” mentioned by Judge Jones came from one single paper Miller discussed at trial, a review article, co-authored by Manyuan Long of the University of Chicago.66 The article’s body text does not even contain the word “information,” much less the phrase “new genetic information.”67
Similar arguments appear in an article co-authored by former NCSE staff member Matzke critiquing critical analysis of evolution. Matzke writes with Paul Gross that it is “scandalously wrong” to argue that modern evolutionary biology has had difficulty accounting for the origin of new biological information because “[c]ompetent scientists know how new genetic information arises.”68 He too relies upon the paper by Long et al. asserting that “it reviews all the mutational processes involved in the origin of new genes and then lists dozens of examples in which research groups have reconstructed the genes’ origins.”69
Most recently, in response to EE on natural selection, the NCSE parrots these claims, claims that “Biologists have no trouble showing how new information (in the sense used by information theorists) originates, nor how new genes, kinds of cells or tissues evolve.”
But are these bold proclamations supported? A closer look shows that the NCSE is equivocating over the meanings of the words “information” and “new,” and that the NCSE’s citations are largely bluffs, revealing little about how new genetic functional information originates via unguided evolutionary mechanisms.
1. The Definition of “Information”
The NCSE would probably define information as probably would define information as “Shannon information,” which means mere complexity. Under this definition, a functionless stretch of randomly garbled junk DNA might have the same amount of “information” as a fully functional gene of the same sequence-length. For example, under Shannon information, which the NCSE would claim is “the sense used by information theorists,” the following two strings contain identical amounts of information:
String A:
SHANNONINFORMATIONISAPOORMEASUREOFBIOLOGICALCOMPLEXITYString B:
JLNUKFPDARKSWUVEYTYKARRBVCLTLODOUUMUEVCRLQTSFFWKJDXSOB
Both String A and String B are composed of exactly 54 characters, and each string has exactly the same amount of Shannon information—about 254 bits.70 Yet clearly String A conveys much more functional information than String B, which was generated using a random character generator.71 For obvious reasons, Shannon complexity has a long history of being criticized as an unhelpful metric of functional biological information.
After all, biological information is finely-tuned to perform a specific biological function, whereas random strings are not. A useful measure of biological information must account for the function of the information, and Shannon information does not take function into account.
Some leading theorists recognize this point. In 2003, Nobel Prize winning origin of life researcher Jack Szostak wrote in a review article in Nature lamenting that the problem with “classical information theory” is that it “does not consider the meaning of a message” and instead defines information “as simply that required to specify, store or transmit the string.”72 According to Szostak, “a new measure of information — functional information — is required” in order to take account of the ability of a given protein sequence to perform a given function. Likewise, a paper in the journal Theoretical Biology and Medical Modelling observes:
[N]either RSC [Random Sequence Complexity] nor OSC [Ordered Sequence Complexity], or any combination of the two, is sufficient to describe the functional complexity observed in living organisms, for neither includes the additional dimension of functionality, which is essential for life. FSC [Functional Sequence Complexity] includes the dimension of functionality. Szostak argued that neither Shannon’s original measure of uncertainty nor the measure of algorithmic complexity are sufficient. Shannon’s classical information theory does not consider the meaning, or function, of a message. Algorithmic complexity fails to account for the observation that “different molecular structures may be functionally equivalent.” For this reason, Szostak suggested that a new measure of information—functional information—is required.73
In 2007 Szostak co-published a paper Proceedings of the National Academy of Sciences with Carnegie Institution origin of life theorist Robert Hazen and other scientists furthering these arguments. Attacking those who insist on measuring biological complexity using the outmoded tools of Shannon information, the authors wrote, “A complexity metric is of little utility unless its conceptual framework and predictive power result in a deeper understanding of the behavior of complex systems.” Thus they “propose to measure the complexity of a system in terms of functional information, the information required to encode a specific function.”74
EE coauthor Stephen C. Meyer follows this approach, writing in a peer-reviewed scientific paper that it is useful to adopt “‘complex specified information’ (CSI) as a synonym for ‘specified complexity’ to help distinguish functional biological information from mere Shannon information—that is, specified complexity from mere complexity.”75 Meyer’s suggested definition of “specified complexity” is useful in describing functional biological information. Specified complexity is a concept derived from the mainstream scientific literature and is not an invention of critics of neo-Darwinism. In 1973, origin of life theorist Leslie Orgel distinguished specified complexity as the hallmark of biological complexity:
[L]iving organisms are distinguished by their specified complexity. Crystals are usually taken as the prototypes of simple, well-specified structures, because they consist of a very large number of identical molecules packed together in a uniform way. Lumps of granite or random mixtures of polymers are examples of structures which are complex but not specified. The crystals fail to qualify as living because they lack complexity; the mixtures of polymers fail to qualify because they lack specificity.76
Orgel thus captures the fact that specified complexity, or CSI, requires both an unlikely sequence and a specific functional arrangement. Specified complexity is a much better measure of biological complexity than Shannon information, a point which the NCSE must resist because it’s much harder to generate specified complexity via Darwinian processes than mere Shannon complexity.
By wrongly implying that Shannon information is the only “sense used by information theorists,” the NCSE avoids answering more difficult questions like how the information in biological systems becomes functional, or in its own words, “useful.” Rather, the NCSE seems more interested in addressing simplistic, trivial questions like how one might add additional characters to a string, or duplicate a string, without regard for the all important question of whether those additional characters convey some new functional message. Since biology is based upon functional information, EE is interested in the far more important question of, Does neo-Darwinism explain how new functional biological information arises?
2. The Computer / Language Analogy
Much like CSI or FSC, EE defines biological information through a comparison to functional computer code:
A large portion of the information needed to construct an organism (with its various traits) is stored in the molecule DNA. Some scientists refer to this information as “assembly instructions” or “a genetic program.” Just like a computer program, DNA contains the biological equivalent of lines of computer code. Evolutionary zoologist Richard Dawkins states, “The machine code of the genes is uncannily computer-like.” (EE, pg. 94)
The NCSE asserts that “Explore Evolution never defines ‘biological information,’ except through error-laden analogies to computers.” While a technical discussion of the various definitions of “information” would probably be beyond EE’s target audience—an introductory biology course—it’s worth noting that the NCSE itself does not define information in its response to EE. This is despite the NCSE’s confident assurances that neo-Darwinism can produce “new information.”
Regardless, EE’s explanation of biological information as “the biological equivalent of lines of computer code” is not only accurate, but seems entirely appropriate and adequate to convey to students the nature of functional biological information. Multiple authorities have compared biological information to computer code, particularly with respect to the fact that changing biological information requires adding new functional lines of code and properly interacting programming components. Not only does Richard Dawkins make the comparison between DNA and computers or an encoded language (see EE’s quote above), but a variety of authorities in scientific journals have done the same.
Hubert Yockey writes in Journal of Theoretical Biology that the comparison of sequence-specific biological information to a symbolic, computer-like language is not a mere analogy, for DNA and language are “mathematically identical”:
[The conservation of biochemistry throughout life] and the universality of the genetic code lead one to believe that life on earth had a beginning and (to use a computer analogy) a basic program of genetic messages originated to form some ancient primitive organism, namely, the protobiont. The biochemical unity of this basic program has been retained throughout evolution in some cases with little modification, and new subroutines have been added. … In the following we will resort to illustrating our points by reference to the properties of language. It is important to understand that we are not reasoning by analogy. The sequence hypothesis applies directly to the protein and the genetic text as well as to written language and therefore the treatment is mathematically identical. … The so-called “instructions in the amino acids themselves” which, it is proposed, generate the first informational biomolecules actually merely play the role of grammar, spelling rules, etc. in ordinary language. Grammar and spelling are autonomous and independent of meaning, so it is clear that it is impossible that the genome of the protobiont could have appeared in a “primitive soup” in this way [a self-organization scenario].77
Writing in the journal Acta Biotheoretica, evolutionary biologist Richard Sternberg offers an extensive and detailed comparison between the information processing ability of the cell to computer programming. Sternberg observes that the genetic code is like computer codes in that it contains the following properties: “Redundancy, error dampening capability, symbolic and semantic flexibility, output versatility, multiple realizability, and text editing.”78 Sternberg continues:
The first use of the term code to look at is one that seems most consonant with the notion many have of g: [DNA language] à [Amino acid language] as a ”genetic program”—as the manner by which data and instructions are represented in a programming (computer-like) language. ORFs are often said to constitute the symbolic text for gene products and the interactions of the latter, and in that sense we are told the genome is software.79
Likewise, one paper in Cell Biology International explains that studying genes is like studying computers:
Genes and emergent gene networks represent programming. These algorithms are written in a pre-existent operating system environment. As in computer science, this language is used by the programmer. We must not only find models for specific genetic programming, but for the genetic operating system. … The algorithmic complexity of life puts our finest computers to shame.80
Another biologist writes in the journal Chaos, Solitons and Fractals that, “Biological function and sign systems, resemble the complexity of computer programs.”81 The NCSE is unjustified in claiming EE makes a “strained analogy” by following authorities like Dawkins and such journal articles which also compare the workings of the genetic code to a computer.
The closest the NCSE comes to defining information comes when it says:
[I]n information theory, adding random noise actually increases the amount of information being transmitted. Whether that information is useful or not to a listener is a separate matter. We usually have a very specific expectation for information transmitted over a telephone line, so random static on the line reduces the amount of information we had hoped to get.
The NCSE’s above commentary fits closely with the standard definition of Shannon information, but the NCSE is wrong when it implies that this is the only way of defining information. Thus, this quote from the NCSE concedes a very important point, namely that such a definition of information is not good at determining “whether that information is useful.”
3. The Definition of “New”
The NCSE would most likely define “new” as merely copying or duplicating some pre-existing stretch of DNA, even if the new copy doesn’t actually do anything new, or perhaps even when the new DNA doesn’t do anything at all. In contrast, proponents of intelligent design would define “new” genetic information as a new stretch of DNA which actually performs some different, useful, and new function. For example, consider the following string:
DUPLICATINGTHISSTRINGDOESNOTGENERATENEWCSI
This 42-character string has ~197 bits of Shannon information. Now consider the following string longer:
DUPLICATINGTHISSTRINGDOESNOTGENERATENEWCSIDUPLICATINGTHISSTRINGDOESNOTGENERATENEWCSI
This procedure just added 42 “new” characters, but no new function has been produced. Assuming there was no way to predict beforehand that the first string would be duplicated just as it was, the amount of Shannon information has doubled, but the amount of CSI has not increased one bit (literally).
The above example is of course analogous to the commonly cited evolutionary mechanism of gene duplication, which evolutionists commonly cite as a mechanism by which Darwinian processes can produce new information. But new functional information is not generated by a process of duplication until mutations change the gene enough to generate a new function—which may or may not be possible. As Professor of Neurosurgery Michael Egnor insightfully said in response to one evolutionary biologist:annon information has doubled, but the amount of CSI has not increased one bit (literally).
The above example is of course analogous to the commonly cited evolutionary mechanism of gene duplication, which evolutionists commonly cite as a mechanism by which Darwinian processes can produce new information. But new functional information is not generated by a process of duplication until mutations change the gene enough to generate a new function—which may or may not be possible. As Professor of Neurosurgery Michael Egnor insightfully said in response to one evolutionary biologist:
[G]ene duplication is, presumably, not to be taken too seriously. If you count copies as new information, you must have a hard time with plagiarism in your classes. All that the miscreant students would have to say is ‘It’s just like gene duplication. Plagiarism is new information- you said so on your blog!’82
Indeed, evolutionary explanations cannot simply rely upon duplication, for there must be duplication followed by recruitment to a new function. However one defines “information,” merely duplicating a string does not produce new functional information.83
4. Finding Darwin in All the Wrong Places
Proponents of neo-Darwinian evolution obscure the fact that they lack explanations for the origin of new functional genetic information through vague appeals to mechanisms such as “gene duplication,” “rearrangement,” and “natural selection.” Such mechanisms are generally inferred from circumstantial evidence, i.e. similarities and differences between gene sequences, where a neo-Darwinian evolutionary history is assumed. More importantly, accounts that invoke such mechanisms almost never attempt to assess the likelihood of mutations producing the genetic changes in question. In this regard, important notes of caution must be observed when assessing evolutionary accounts of the origin of a gene.
A 2007 article by evolutionary biologist Michael Lynch in Proceedings of the National Academy of Sciences USA goes to the heart of some of the assumptions inherent in many claims of neo-Darwinian evolution. Lynch provides a list of myths promoted by biologists, and he calls it a “myth” to believe that “Characterization of interspecific differences at the molecular and/or cellular levels is tantamount to identifying the mechanisms of evolution.”84
Of course, one of the typical “mechanisms of evolution” cited is natural selection, commonly invoked to account for how a gene duplicate acquires a new function. But what kind of evidence is sufficient to demonstrate that positive selection, or natural selection acting to preserve adaptive mutations, has occurred? Biologist Austin L. Hughes warns that most inferences of positive selection are based upon questionable statistical analyses of genes:
A major hindrance to progress has been confusion regarding the role of positive (Darwinian) selection, i.e., natural selection favoring adaptive mutations. In particular, problems have arisen from the widespread use of certain poorly conceived statistical methods to test for positive selection. Thousands of papers are published every year claiming evidence of adaptive evolution on the basis of computational analyses alone, with no evidence whatsoever regarding the phenotypic effects of allegedly adaptive mutations. … Contrary to a widespread impression, natural selection does not leave any unambiguous ”signature” on the genome, certainly not one that is still detectable after tens or hundreds of millions of years. To biologists schooled in Neo-Darwinian thought processes, it is virtually axiomatic that any adaptive change must have been fixed as a result of natural selection. But it is important to remember that reality can be more complicated than simplistic textbook scenarios. … In recent years the literature of evolutionary biology has been glutted with extravagant claims of positive selection on the basis of computational analyses alone … This vast outpouring of pseudo-Darwinian hype has been genuinely harmful to the credibility of evolutionary biology as a science.85
In short, evolutionary biologists commonly assume that mutations that change protein sequence were fixed by natural selection, but this assumption may not hold true since many such mutations are neutral and confer no selective advantage.
Biochemist Michael Behe offers another reason not to infer neo-Darwinian mechanisms of change based upon mere evidence of sequence similarity:
Although useful for determining lines of descent … comparing sequences cannot show how a complex biochemical system achieved its function—the question that most concerns us in this book. By way of analogy, the instruction manuals for two different models of computer put out by the same company might have many identical words, sentences, and even paragraphs, suggesting a common ancestry (perhaps the same author wrote both manuals), but comparing the sequences of letters in the instruction manuals will never tell us if a computer can be produced step-by-step starting from a typewriter. … Like the sequence analysts, I believe the evidence strongly supports common descent. But the root question remains unanswered: What has caused complex systems to form?86
[M]odern Darwinists point to evidence of common descent and erroneously assume it to be evidence of the power of random mutation.87
Many scientific papers purporting to show the evolution of “new genetic information” do little more than identify molecular similarities and differences between existing genes and then tell evolutionary just-so stories of duplication, rearrangement, and subsequent divergence based upon vague appeals to “positive selection” that purport to explain how the gene arose. But exactly how the gene arose is never explained. In particular, whether chance mutations and unguided natural selection are sufficient to produce the relevant genetic changes is almost never assessed.88 These scientific papers play the Gene Evolution Game, an easy game to play, as we’ll see in Part 4 below.
5. How to Play the Gene Evolution Game (note: This section is written tongue-in-cheek)
The Gene Evolution Game is a very simple game to play. In three examples, we’ll develop three rules that can help you explain the origin of any new gene. That’s right—any gene! Let’s start with a simple example:
Rule 1: The Magic Wand of Gene Duplication
Where do new genes come from? Gene duplication is typically how we explain where a new gene comes from. Here’s how it works, in 4 easy steps:
(1) Take a gene you’ve observed in some organism. We’ll call it Gene B.
(2) Find another gene similar to Gene B. Let’s call it Gene A.
(3) Claim that at some time in the past, Gene A duplicated so then there were two copies of Gene A.
(4) Then assert that one of Gene A’s duplicates evolved into Gene B.
Gene duplication is thus very a powerful explanation, and it looks like this:
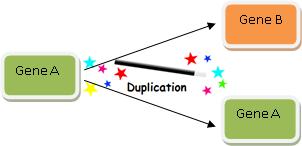
Wasn’t that easy? We’ve just explained how Gene B evolved! So when you find two genes with high sequence similarity, you can always explain how one evolved from the other via the magic wand of Gene Duplication.

The NCSE says “Gene duplication are [sic] common events, resulting from small errors in the process of cell replication. Once a gene is duplicated it is possible for one copy to mutate, adding information without risking the functioning of the pre-existing gene.” That’s all you need to know—when you invoke duplication, you needn’t worry about whether there is some functional evolutionary pathway for the duplicate gene to follow as it acquires some new function. In other words, you don’t need to worry about how new functional genetic information arises because “gene duplication” explains everything worth explaining! It’s easy to get extra genetic information in the Shannon sense, and that’s all that matters.
Rule 2: No Worries—Natural Selection Can Do It!
Now obviously the modern version of Gene B we find doesn’t perfectly resemble Gene A, or else it would be Gene A. So we have to account for how a copy of Gene A acquired its new function—Function B. One might think this would be the key part of explaining how new functional genetic information arises, but believe it or not, this is actually the easiest and quickest aspect of the game: we just call upon the power of “natural selection” and the problem is solved! This diagram shows exactly how we do it:
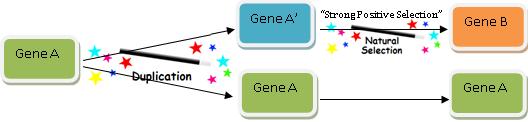
The great thing about the Gene Evolution Game is that natural selection can change (or not change, depending on what you wish) almost anything. And I mean anything.
Don’t worry about the details. If you want to account for differences between Gene B and Gene A, natural selection is always up to the challenge. Don’t worry about whether Gene A’ could evolve from Function A to Function B by small sequential adaptive steps. Don’t worry about the order in which amino acids changed, or whether many mutations were necessary to gain any functional advantage (that sort of thing is too unlikely to occur anyway, so just ignore it). Don’t worry about adaptive constraints, weak selection, or loss due to genetic drift. And most of all, definitely don’t do any calculations to determine the likelihood of whether all of the changes could have occurred in any reasonable amount of time.
We know the gene must have evolved, therefore it did evolve. Thus, you can think of natural selection as another magic wand. It may be invoked at any time to explain how a gene changed or evolved to acquire its new function.

This wand is a very powerful tool—it can explain both why things change, and why things stay the same. Wow!89
Rule 3: The Magic Wand of “Rearrangement”
To play the Gene Evolution Game, there’s one last trick you need to know. Sometimes Gene B isn’t similar to just Gene A. Sometimes part of Gene B looks like Gene A, but another part looks like another gene. We’ll call the latter Gene Z. Don’t worry—this is all still easy to explain! We start by invoking duplication: Imagine that Gene A and Gene Z both duplicated, and then both duplicate copies were suddenly transported across the genome so that now they reside on a chromosome right next to one another. This is called “rearrangement.” If this sounds a little complicated, we’ll draw some diagrams to show how it works:
Step 1: Gene A and Gene Z are each in different locations, maybe even on different chromosomes:
Then a special process called “rearrangement” suddenly rearranges and transports Gene A and Gene Z so they’re right next to each other in some other location in the genome. Rearrangement is a powerful magic wand you can invoke to explain how two stretches of DNA that initially are far apart suddenly end up near one another. They then can form a new functional gene. You’re probably getting a feel by now for how this works:

There are all kinds of rearrangements you can invoke—insertions, deletions, inversions, translocations—and you can invoke them in virtually any order and in any amount you please to explain how you get any two, or three, or even dozens of pieces of DNA to come together from throughout the genome to end up right next to one-another so that presto, you have your new functional gene. Just mix and match these types of rearrangements as needed to create virtually whatever DNA sequence you desire—rearrangement is always up to the task.

It’s all downhill from here. Natural selection can then perfect the rearranged gene to make it functional. Never mind detailed demonstrations that this actually works. Just sprinkle some natural selection and Gene A and Gene Z will magically combine functions and evolve into Gene B. Here we go, completing the explanation with everything we need to know:

Using the three magic wands of duplication, rearrangement, and natural selection you can provide a full and complete detailed explanation for the evolution of virtually any gene.
No Identifiable Ancestor? No Worries!
First, in some cases, your gene (i.e., Gene B) only has a homologue known from an entirely different species. So how did Gene B arrive in your organism? In these cases, just invoke lateral gene transfer (LGT) to whoosh the right gene into your organism. It doesn’t even matter whether lateral gene transfer is thought to occur between the organisms you’re working with—if the gene you need is found in some other species, then that by itself is evidence that lateral gene transfer occurs between the organisms you’re working with!90
Second, sometimes part of your gene doesn’t resemble part of any other known gene anywhere. Some people might wonder, “Where did this gene come from?” You still don’t worry about this. Remember what we said about natural selection? It can change anything.
So if you can’t find any similar genes, just assume that your unique DNA sequence has evolved so much due to natural selection that it just doesn’t resemble its ancestral sequence any longer. But don’t worry, it’s not, and never is, the case that there wasn’t an ancestor. It’s just that the strong powers of natural selection changed the gene so much that we can’t identify any possible ancestral sequence.91
Some Final “Do’s” and “Don’ts” of the Gene Evolution Game
Right about now, you might be wondering about that last example we gave. So before you go any further, here’s a reminder of some questions you don’t need to ask:
- Given the known effects and rates of mutations, what were the odds of Gene A and Gene Z suddenly being rearranged next to one-another so that they could now function together as one single new gene product, Gene B?
- Did the rearranged gene product B start out functional? If not, how quickly could it gain function? How was it preserved from loss until it became functional?
- Are proteins really as malleable as this story would suppose or would the new combined gene encounter folding or other contextual problems?
- What mutational pathway was taken to evolve Gene A and Gene Z into a new gene with function B?
- What selective advantages were gained at each small step of this evolutionary pathway?
- Were any “large steps” (i.e., multiple specific mutations) ever required to gain a selective advantage along the evolutionary pathway? Would such “large steps” be likely to occur?
- Could all of this happen on a reasonable timescale?
You don’t need to worry about these questions. In fact, believe it or not, you don’t even need to know the function of your gene to claim it evolved from A and Z! All you need to know is that Genes A, Z, and B exist. This summary of these 3 simple rules of the Gene Evolution Game will help you explain anything:
Gene Evolution Game Rule 1: Whenever you find sequence homology between two genes, just invoke a duplication event of some hypothetical, ancient ancestral gene, and you can explain how two different genes came to share their similarities.
Gene Evolution Game Rule 2: When you need to explain how a gene acquired some new function, or evolved differences from another gene, just invoke the magic wand of natural selection. No need to demonstrate that there is any benefit to the new gene, or that a step-wise path to adaptation exists. Finally, natural selection is especially useful when part of your gene appears unique—since natural selection can change anything, just conclude that natural selection changed your gene so much that it no longer resembles its ancestor.
Gene Evolution Game Rule 3: When a gene seems to be composed of the parts of several genes, just invoke duplications and rearrangements of all the DNA sequences you need, so you can get them all together in the right place. If you need to delete parts of a gene, or invert them, or transpose to a new location, just invoke different types of rearrangements as often and as liberally as you wish, and ba-da-bing, you’ve got your new gene!
And remember, don’t ask those other hard questions. Just use these three rules and you can explain virtually anything. No details required!
6. Asking the Right Questions
On a more serious note, it’s easy to duplicate a gene, but the key missing ingredient in many neo-Darwinian explanations of the origin of new genetic information is how a gene duplicate then acquires some new optimized function. Evolutionists have not demonstrated, except in rare cases, that step-wise paths to new function for duplicate genes exist.
As we saw earlier, Austin Hughes cautions against making “statistically based claim[s] of evidence for positive selection divorced from any biological mechanism.”92 In other words, natural selection is invoked to explain the evolution of genes where we do not even know the functional effect of the mutations being asserted. In this regard, Hughes observes that even in one of the more sophisticated studies, “there was no direct evidence that natural selection was actually involved in fixing adaptive changes.”93
Hughes also acknowledges a problem inherent in many appeals to natural selection, namely that required mutations may not give any selective advantage when they first arise. He thus writes regarding one study:
For example, a rhodopsin from the Japanese conger eel with λmax ≈ 480 nm achieved this sensitivity through the interaction of three different amino acid replacements (at sites 195, 195, and 292). There does not seem to be any way that natural selection could favor an amino acid replacement that would be of adaptive value only if two other replacements were to occur as well.94
In this case, there was no stepwise advantage gained with each successive mutation.
Because no advantage could have been gained until all three mutations were present, Hughes finds it more “plausible” to believe that the first two mutations were “selectively neutral” and became fixed due to random, non-adaptive processes such as genetic drift.
Once the third mutation arose it might have provided an advantage, but to paraphrase Scott Gilbert, at best this really only explains the survival of the fittest, not the arrival of the fittest.95
But Hughes’ explanation has deep deficiencies: it requires that two mutations become fixed before any selective advantage for the third mutation is gained. This implies that there must be three specific mutations to gain any selective advantage. A key question is thus, Are multiple specific mutational changes likely to appear in the same individual through unguided chance mutations given known mutation rates and population sizes? Even Hughes, despite his exhortations to fellow evolutionary biologists to employ more rigor in their studies, does not address this fundamental question.
A similar example is found when leading paleoanthropologist Bernard Wood critiqued a simplistic model of human cranial evolution on the grounds that too many mutations would be required to gain any functional advantage:
The mutation would have reduced the Darwinian fitness of those individuals. . . . It only would’ve become fixed if it coincided with mutations that reduced tooth size, jaw size and increased brain size. What are the chances of that?96
Similarly, Jerry Coyne writes that “It is indeed true that natural selection cannot build any feature in which intermediate steps do not confer a net benefit on the organism.”97 This highlights a key deficiency in many neo-Darwinian accounts of the evolution of genes.
Namely, they fail to demonstrate that the processes necessary to generate new functionally advantageous genetic information are plausible. As with Hughes’s or Wood’s examples above, multiple mutations might be necessary to gain any functional advantage. Any account invoking blind, unguided, random mutations to evolve a gene from Function A to Function B must address at least these three questions:
- Question 1: Is there a step-wise adaptive pathway to mutate from A to B, with a selective advantage gained at each small step of the pathway?
- Question 2: If not, are multiple specific mutations ever necessary to gain or improve function?
- Question 3: If so, are such multi-mutation events likely to occur given the available probabilistic resources?
Mathematician David Berlinski considers such questions when critiquing evolutionary accounts of eye evolution. Darwinian processes fail because multiple changes are required for a new function to appear:
If these changes come about simultaneously, it makes no sense to talk of a gradual ascent of Mount Improbable. If they do not come about simultaneously, it is not clear why they should come about at all.98
Again, the key question is therefore, how hard is it for new functional biological information to arise? Answering this question requires assessing the ability of random mutation and natural selection to generate new functional biological information. But when most evolutionary biologists play the Gene Evolution Game, they do not make such assessments and rarely consider these questions. Instead they typically invoke processes such as gene duplication, natural selection, and rearrangement, without demonstrating that random and unguided mutations are sufficient to produce the information needed. Any explanation that at base is little more complicated than “duplication, rearrangement, and natural selection” is not a demonstration that new functional genes can arise by unguided processes.
Thankfully, some scientists are willing to consider these key questions. They have performed research providing data that offers strong reasons to be skeptical of the ability of mutation and selection to form new functional genetic sequences.
i. Asking Questions 1 and 2:
Molecular biologist Doug Axe has performed mutational sensitivity tests on enzymes and found that functional protein folds may be as rare as 1 in 1077.99 His research shows that the fitness landscape for many enzymes looks like this, making it very unlikely that neo-Darwinian processes will find the specific amino acid sequences that yield functional protein folds:
To put the matter in perspective, these results indicate that the odds of Darwinian processes generating a functional protein fold are less than the odds of someone closing his eyes and firing an arrow into the Milky Way galaxy, and hitting one pre-selected atom.100 To say the least, this exhausts the probabilistic resources available. Such data help us answer the first question: it’s not likely that there will be a functional stepwise mutational pathway leading from Function A to Function B.
Douglas Axe is by no means the only biologist to make this observation. A leading college-level biology textbook, Campbell’s Biology, observes that “Even a slight change in primary structure can affect a protein’s conformation and ability to function.”101 Likewise, David S. Goodsell, an evolutionist biologist, writes:
As you might imagine, only a small fraction of the possible combinations of amino acids will fold spontaneously into a stable structure. If you make a protein with a random sequence of amino acids, chances are that it will only form a gooey tangle when placed in water. Cells have perfected the sequences of amino acids over many years of evolutionary selection…102
What Goodsell does not mention is that if “perfected” amino acid sequences and functional protein folds are rare and slight changes can disrupt function, then selection will be highly unlikely to take proteins from one functional fold to the next without traversing some non-functional stage. So how do new functional protein folds evolve? This effectively answers question two, implying that many specific mutations would be necessary for evolving genes to pass through non-functional stages while evolving some new function. Question 3 assesses whether this is likely to happen.
ii. Asking Question 3:
In 2004, Michael Behe and physicist David Snoke published a paper in the journal Protein Science reporting results of computer simulations and theoretical calculations. They showed that the Darwinian evolution of a simple functional bond between two proteins would be highly unlikely to occur in populations of multicellular organisms. The reason, simply put, is because too many amino acids would have to be fixed by non-adaptive mutations before gaining any functional binding interaction. They found:
The fact that very large population sizes—109 or greater—are required to build even a minimal [multi-residue] feature requiring two nucleotide alterations within 108 generations by the processes described in our model, and that enormous population sizes are required for more complex features or shorter times, seems to indicate that the mechanism of gene duplication and point mutation alone would be ineffective, at least for multicellular diploid species, because few multicellular species reach the required population sizes.103
According to this data, chance mutations are unlikely to produce even two required non-adaptive mutations in multicellular diploid species within any reasonable timescale. This answers the third question: getting multiple specific non-adaptive mutations in one individual is extremely difficult, and more than two required but non-adaptive mutations are likely beyond the reach of multi-cellular organisms. Studies like this show that the actual ability of random mutation and unguided selection to produce even modestly complex new genetic functions is insufficient.
In 2008, Behe and Snoke’s would-be critics tried to refute them in the journal Genetics, but found that to obtain only two specific mutations via Darwinian evolution “for humans with a much smaller effective population size, this type of change would take > 100 million years.” The critics admitted this was “very unlikely to occur on a reasonable timescale.”104 In other words, there is too much complex and specified information in many proteins and enzymes to be generated in humans by Darwinian processes on a reasonable evolutionary timescale.
As noted in the comments on the Gene Evolution Game, when neo-Darwinists try to explain the evolution of genes, mere point mutations often are insufficient to account for the gene’s sequence. They must therefore appeal to genetic rearrangements such as insertions, deletions, or an alleged process called “domain shuffling” where segments of proteins become shuffled to new positions in the genome. In his book The Edge of Evolution, Michael Behe reviews research that engineered new protein function by swapping domains to change protein function, and found that the intelligently engineered changes required multiple modifications that, in nature, would require too many simultaneous mutational events to yield functional changes:
[Protein engineering research] does not mimic random mutation. It is the exact opposite of random mutation. … What do the lab results tell us about whether random-yet-productive shuffling of domains “occurs with significant frequency under conditions that are likely to occur in nature”? About whether that is biologically reasonable? Nothing at all. When a scientist intentionally arranges fragments of genes in order to maximize the chances of their interacting productively, he has left Darwin far, far behind. … [Experiments that engineered proteins by shuffling domains] didn’t just splice two genes together in a single step; they took several additional steps as well. … Remember the more steps that have to occur between beneficial states, the much less plausible are Darwinian explanations. … Domain shuffling would be an instance of the “natural genetic engineering” championed by James Shapiro where evolution by big random changes is hoped to do what evolution by small random changes can’t. But random is random. No matter if a monkey is rearranging single letters or whole chapters, incoherence plagues every step. … One step might luckily be helpful on occasion, maybe rarely a second step might build on it. But Darwinian processes in particular and unintelligent ones in general don’t build coherent systems. So it is biologically most reasonable to conclude that, like multiple brand new protein-protein binding sites, the arrangement of multiple genetic elements into sophisticated logic circuits similar to those of computers is also well beyond the edge of Darwinian evolution.105
As Behe observes, “No matter if a monkey is rearranging single letters or whole chapters, incoherence plagues every step.” Thus, when multiple mutational events—whether point mutations, “domain shuffling,” or other types of rearrangements—are required to gain some functional advantage, it seems unlikely that blind neo-Darwinian processes can produce the new biological function.
Unfortunately, few if any advocates of the neo-Darwinian just-so stories investigate whether mutation and natural selection are sufficient to produce new functional genetic information. Instead they believe that finding similarities and differences between genes demonstrates that neo-Darwinian evolution has occurred, and they assume that “positive selection” is a sufficient explanation.
As Hughes cautions, they engage in “use of certain poorly conceived statistical methods to test for positive selection,” causing “the literature of evolutionary biology [to become] glutted with extravagant claims of positive selection” resulting in a “vast outpouring of pseudo-Darwinian hype [that] has been genuinely harmful to the credibility of evolutionary biology as a science.”106 Or, as Michael Behe cautions, they confuse mere sequence similarity with evidence of neo-Darwinian evolution. Finally, Michael Lynch warns his colleagues that “Evolutionary biology is not a story-telling exercise, and the goal of population genetics is not to be inspiring, but to be explanatory.”107
With these principles in mind, we will assess about a dozen of the just-so stories concerning the origin of genes offered in studies cited by the NCSE.
7. Assessment of the NCSE’s Citation of Long et al.
In its response to EE on natural selection, the NCSE cites a review paper co-authored by Manyuan Long108 claiming the paper describes “well-studied examples of recently evolved genes, and a summary of what scientists have learned about the processes by which those genes evolved.” The NCSE wants its readers to believe that Long et al. demonstrates the origin of new biological information by Darwinian processes. In fact, what Long et al. actually demonstrates is that neo-Darwinists do not want to ask the right questions—the hard questions—about the sufficiency of their theory to explain gene evolution. They accept superficial just-so stories in place of detailed, plausibly demonstrated explanations.
Just as in the Gene Evolution Game, the studies cited in the review by Long et al. repeatedly invoke gene duplication, natural selection, and genetic rearrangements. But many offer little more than vague just-so stories that commit the mistakes Lynch warns of—mistaking story-telling for explanation. In fact, many of these accounts barely rise to the level of “explanation.”
To show how heavily the NCSE relies on Long et al. in its response to Explore Evolution, let’s look at how the NCSE reproduces a lengthy table (Table 2) from Long et al. The table lists a number of genes whose evolutionary origin has supposedly been explained.109 Many of the examples from this Table 2 are mere story-telling exercises based upon assumptions which do not explain or answer deeper questions about how neo-Darwinian evolution generates new functional genetic information:
a. Jingwei
The first entry in the table comes from a study that Long co-authored with Charles Langley in Science. The study asserts that a fruit fly gene, jingwei, arose when part of another gene, Adh, was retrotransposed into a new location on a fruit fly chromosome near a duplicate of the gene yellow-emperor.110 Their evidence for this rearrangement is sequence similarity between part of jingwei and Adh, and part of jingwei and yellow-emperor. Thus, invoking Gene Evolution Game Rules 1 and 3, the authors tell a story that presumes that hypothetical duplicates of yellow-emperor and Adh were fortuitously spliced together to create a new functional gene—jingwei. The exact word used is that exons were “recruited” from elsewhere into the genome “by capturing several upstream exons and introns of an unrelated gene” to produce “a new functional gene.” They author make no attempt to address the more important questions, such as whether a step-wise path to such a genomic rearrangement could have happened by unguided chance to fortuitously produce this gene. Merely finding sequence similarity between exons and other genes (or pseudogenes) does not thereby demonstrate neo-Darwinian evolution.
Long et al. claim that jingwei is only 2.5 million years old, but the original study compared the Adh-like exon in jingwei with the allegedly ancestral exon from Adh and found that they were so different that they must have diverged at least 30 million years ago. This poses a problem, because this fruit fly clade is not thought to be nearly that old; as Long and Langley write, “This conflicts with the age of the melanogaster subgroup, which is estimated to be 17 to 20 million years.” More important, the unexpectedly high degree of differences between the exons is taken, under neo-Darwinian assumptions, as evidence that jingwei “responded to positive natural selection and evolved a new function.” Yet according to one commentator, despite the fact that they are sure natural selection drove this gene to acquire its new function, “its actual function is obscure.”111 So they claim that natural selection was the driving mechanism, but they do not even know for sure in this paper that the gene has a function. They have not addressed any of the deeper questions of gene evolution, instead offering an incomplete and assumption-based story that ignores warnings from Austin Hughes against invoking “positive selection divorced from any biological mechanism.”112
b. Sdic
A second study cited by Table 2 asserts that various genes were duplicated, parts of which were then fused to create a new gene “de novo.”113 The authors wanted to explain how part of one gene, Cdic, became fused with part of another gene, Annx, but they ran into problems because the genes exist on the chromosome in a different order from the gene being studied.
Making complicated use of Rules 1 and 3 of the Gene Evolution Game, they speculate that there was a series of duplications and rearrangements—highly selective and specific deletions—and then more duplications to produce this gene. This included one non-coding region spontaneously becoming a coding region, termed the de novo origin of a gene. After this complicated story, the paper concludes that Sdic arose from “extensive refashioning” of the genome.
Of course, it is also necessary to explain the origin of the promoter region of this gene, about which the authors state:
First, although a testes-specific promoter was essential for Sdic, this unusual regulatory region did not really “evolve.” Instead it was aboriginal, created de novo by the fortuitous juxtaposition of suitable sequences. The more extensive evolutionary changes took place in Cdic intron 3, enabling an originally untranslatable sequence to become a new coding region whose product functions in the assembly of axonemal dynein.114
This “de novo” origin of a functional gene is an event that even Long et al. admits is “rare.”115
The authors then invoke strong positive selection due to the unlikelihood that such a dramatic reorganization “would have originated and been maintained in the absence of positive selection.”
Despite their appeal to positive selection, the authors admit they aren’t even sure exactly what the gene does, stating: “We do not yet know how Sdic contributes to the function of the sperm axoneme, or even whether it is essential for male fertility.” So once again, they are sure it evolved due to “positive selection” but they do not even know exactly what function was being selected for.
A gene’s being “created de novo by the fortuitous juxtaposition of suitable sequences,” a mechanism that is “rare,” is not a compelling evolutionary explanation. This incomplete just-so story vaguely appeals to multiple mutations without assessing whether they would be likely to occur or what advantage they are offering. The story is no explanation at all.
c. Cid
The authors of this paper studied nucleotide differences between Cid genes in two closely related fruit fly species and found that nucleotide differences that led to changes in amino acid sequence were nearly 10 times more common than “silent” differences that did not affect amino acid sequence.116 Using Darwinian assumptions and Gene Evolution Game Rule 2, this led the authors to conclude that there was positive selection pressure on the gene to evolve.
Yet in this study natural selection was invoked not only to explain how genes changed, but also how genes stayed the same: a low number of replacement changes were taken as evidence of a “selective sweep,” a strong purifying selection that weeded out variation, to prevent change in one lineage. Thus, both a high degree of amino-acid changing differences and a low degree of amino-acid changing differences were taken as evidence of natural selection. Whether any of this is correct is purely a matter of ad hoc inference and starting assumptions. Moreover, the authors provided no mutation-by-mutation account to explain the selective advantages (or lack therefore) that might have been generated by any amino acid changes.
In light of the study’s methodology, Michael Lynch’s warning now comes to mind. It is a “myth” to believe that “[c]haracterization of interspecific differences at the molecular and/or cellular levels is tantamount to identifying the mechanisms of evolution.” Additionally, this study violates Austin Hughes’s admonition against “the widespread use of certain poorly conceived statistical methods to test for positive selection” which have caused “the literature of evolutionary biology [to become] glutted with extravagant claims of positive selection on the basis of computational analyses alone” resulting in a “vast outpouring of pseudo-Darwinian hype [that] has been genuinely harmful to the credibility of evolutionary biology as a science.”117 It’s also noteworthy that this study merely investigated how variations of the same gene originated in two closely related species, not how a new gene originated in the first place.
d. Arctic AFGP and Antarctic AFGP
Two papers cited by Table 2 in Long et al. discuss the origin of antifreeze genes (AFGP) in species of Arctic and Antarctic fish. The two species have similar antifreeze genes, even though they exist on literally opposite sides of the globe and are only distantly related. For the neo-Darwinist, these findings require that “near-identical antifreeze glycoproteins”118 evolved independently in distantly related species of fish—one in the Arctic and another in the Antarctic—via what is called “a striking case of convergent evolution.”119
Employing Gene Evolution Game Rules 1 and 3, a paper commenting on this research states the genes arose by “[d]uplication, divergence, and exon shuffling” and were “cobbled together from DNA of no related function (or no function at all).”120 For key parts of the antifreeze gene in Arctic cod, the commentators noted that the investigators “did not find any database matches to the sequence”121 and therefore could not determine its origin.
However, there were matches for the Antarctic AFGP sequence, where similarities were found with part of a trypsinogen gene. This led to speculation about an evolutionary scheme that started with a trypsinogen gene, most of which was then deleted, followed by “recruitment” of a short threonine-alanine-alanine coding element, which then led to “de novo amplification of a short DNA sequence to spawn a novel protein with a new function.”122
This “de novo amplification of the coding element gave rise to an entirely new coding region that encodes the repetitive tripeptide backbone of AFGP,” even though this key component had “arisen (in part) from noncoding DNA.”123 Thus, according to their story, non-coding DNA spontaneously became functional and was duplicated many times to create the core functional “backbone” of this gene. No attempt was made to assess the mutational odds of such DNA that has “no function at all” suddenly becoming a key functional component of this gene.
This evolutionary story also solves problems through vague appeals to Gene Evolution Game Rule 2. The many genetic changes necessary to suddenly create this functional antifreeze gene were apparently accounted for by simply appealing to “powerful environmental selectional pressure” due to the need of the fish to survive in cold water.124 Of course, no statistical analyses were performed to assess the likelihood of cobbling together functional genes from completely unrelated stretches of DNA, some of which was previously non-functional, to produce a new functional antifreeze gene. Rather, one paper simply asserted the “creative” power of “molecular mechanisms”:
To consider the AFGP story as a special case of duplication and divergence would be oversimplifying; it is clear that the antifreeze function, or even a related function that could be converted to the purpose, was not present in trypsinogen. The molecular mechanisms involved in the formation of this gene were indeed more creative—making sense from nonsense—by calling into a functional coding capacity intronic DNA sequences.125
Are these molecular mechanisms likely to produce this gene? Are random mutations likely to “mak[e] sense from nonsense”? No analysis was given. The antifreeze genes are polyproteins, meaning they are complex many-in-one proteins designed to be cut into many pieces of specific lengths, each of which performs an important antifreeze function. The different segments are separated by special separator markers and cleaved by a specific protease. In this regard, no analysis was given to account for the origin of associated cleaver protease enzymes necessary for the function of the AFGP gene.
These papers base their claims of evolution purely upon circumstantial evidence—comparisons of sequence similarity—and then tell a tale of deletion, reshuffling, and amplification. Explanation of these genes by “cobbling” via “[d]uplication, divergence, and exon shuffling” and “de novo” recruitment of non-coding sequences does not account for how such a complex gene could actually originate. This story does not address how the complex many-proteins-in-one nature of these proteins evolved, nor was any consideration given the odds of spontaneously producing this functional gene. Nor have these investigators explained the highly unlikely event that two species would independently evolve highly similar antifreeze proteins.
The antifreeze proteins are highly repetitive, and may have less specified complexity than most proteins.
Nonetheless, there’s no real evidence for neo-Darwinian evolution here, only sequence comparisons and a lot of missing details.
e. Adh-Finnegan
This article cited by Long et al. represents an example where a stretch of DNA that was previously presumed to be a “nonfunctional” pseudogene turned out to be a functional gene.126 The functional gene was then named Adh-Finnegan after “Tim Finnegan, a character from an Irish folksong, [who] was mistakenly declared dead and subsequently arose during his own wake.” This is a good example of how the junk-DNA myth initially led scientists to the wrong conclusion about this gene.
This paper’s just-so story makes use of all three rules of the Gene Evolution Game. Despite its citation in Long et al. (and thus by the NCSE), the study sheds very little light on the origin of the gene in question, other than to claim it evolved from another highly similar Adh gene and then “recruited” sequences via rearrangement from elsewhere in the genome.
Predictably, an ancient duplication event is invoked to account for the origin of the gene, and then selection is invoked as a magic wand to account for “radical change in the structure” of the gene “compared to that of its highly conserved Adh ancestor.”
Extensive rearrangements are also invoked to explain how the gene “recruited ~60 new N-terminal amino acids,” as well as “the acquisition of new amino acid residues upstream from the ancestral ATG initiation codon.” The origin of the N-terminal exon posed a problem, however, because “A database search revealed no similarity of the N-terminal exon to known proteins,” and thus as Long et al. note, the gene must have “[r]ecruited a peptide from an unknown souce [sic].” The author claims that a “rapid rate of evolution” of the exon prevented its identification.
Thus, the paper concludes: “For the moment we will posit that a genomic rearrangement (perhaps resulting from unequal crossing over) juxtaposed the first exon from an unknown donor gene to the 5′-flanking region of the ancestor of Adh-ψ.” The mutational odds of suddenly rearranging these stretches of DNA into one place to compose a functional gene are never considered.
Ignoring the warnings of Austin Hughes, the author asserted, incredibly, that there was “rapid, adaptive evolution” and that “positive selection has played an important role in the evolution” of this gene even though the function of the gene is not known.
f. FOXP2
This gene is commonly cited as being involved in the origin of human language, even though it’s not exactly clear what it does.127 In fact, one study observed that “The finding that FOXP2 is critical to speech and language does not by itself demonstrate the role of this gene in the origin of human speech, because the function of FOXP2 could have remained unchanged during human evolution while other speech-related genes changed.”128
The studies cited by Long et al. compared human FOXP2 to copies of the same gene in chimps, gorillas, orangutans, the macaque, and mice, and found that “FOXP2 is a conserved protein, with only three amino acid differences (and a 1-amino-acid insertion/deletion) between human and mouse in its entire length of 715 amino acids.”129 Thus, this paper did not really study the origin of a new gene, but only tried to explain how human FOXP2 obtained a mere two differences in amino acid sequence from FOXP2 in apes.
In this case, the high ratio of non-synonymous (i.e. amino acid changing) to synonymous (i.e. silent) nucleotide differences was taken as evidence of the force of “positive selection.”130
Again, selection is being inferred, even though the authors didn’t know exactly what the gene does, violating Austin Hughes’s warning against “statistically based claim[s] of evidence for positive selection divorced from any biological mechanism.”131 At base, these studies catalogued interspecific differences between human FOXP2 and FOXP2 from other species, and found that those differences were extremely slight. Even if neo-Darwinian mechanisms were indeed at work, the degree of evolution in human FOXP2 amounts to 2 mutations, and 2 amino acid changes. This is an interesting finding, but not useful in explaining actually noteworthy or impressive degrees of genetic evolution.
g. Cytochrome c1
This paper sought to explain the origin of a gene, cytochrome c1, involved in energy production in plants.132 The study found sequence similarity between three exons in cytochrome c1, a gene that operates in the mitochondria, with a gene with a very different function, GapC, which operates in the cytoplasm.133 That sequence similarity, essentially, formed the entire basis for this evolutionary story of rearrangement of exons, which made heavy use of Gene Evolution Game Rule 3. Since cytochrome c1 is less widespread than Gapc1, the authors concluded that Gapc1 is older and therefore “donated” the exons to cytochrome c1 through “exon shuffling.” Additionally, they speculate that the ancestral cytochrome c1 gene had the same function, but these new exons (for some reason) allowed the same function to be performed—but even more efficiently: “The ancestral cytochrome c1 gene in plants must have been targeted to the mitochondrion; thus this targeting sequence was replaced in the line leading to the potato by the GapC gene. This replacement may have been selected by some advantage in using the GapC promoter.”
Predictably, the authors never discuss the mutational odds of replacing exons in one gene with exons “donated” from another gene such that the gene not only remains functional but has an advantage in performing its original function. This is the key phase where new genetic information must arise, but the authors never assess whether it would be likely to occur via unguided mutations.
h. Morpheus
This study aimed to explain the origin of a group of genes named morpheus that had changed so much that their origin could not be traced to any other gene. As the paper lamented, “some genes emerge and evolve very rapidly, generating copies that bear little similarity to their ancestral precursors” and thus “may not possess discernable orthologues within the genomes of model organisms.”134 When studying these genes, they reported “no significant sequence similarity to this gene family in other organisms at either the nucleotide or protein level.” Since it was impossible to invoke a scheme of duplications or other rearrangements from which this genetic material found its origin, the authors simply concluded, “These data suggested that the exonic regions were hypermutable or that amino-acid changes had been selected during the evolution of this gene family” and that their “analysis has revealed an extraordinary degree of evolutionary plasticity.” In other words, they have no idea where this gene came from, so they invoke the claim that the genes were “hypermutable” and subject to strong selection pressure such that their origin cannot be traced. How the genes actually arose is a question the authors never really address. Incredibly, they again appeal to strong selection pressure despite admitting “the precise function of this gene family is unknown.” Gene Evolution Game Rule 2 solved all the problems without anyone’s having to investigate the plausibility of the mechanism.
i. TRE2
This paper invoked “the chimeric fusion of two genes” to explain how the gene Tre2 evolved from duplicates of two other genes.135 The story is simple: Tre2 has 30 exons: exons 1-14 appear similar to another gene, TBCID3, while exons 15-30 are similar to the gene USP32.
Thus the authors characterized the origin of this gene as “the abrupt creation of a mosaic gene with novel functions.” Although the authors claim that “domain accretion and gene-fusion events may not be uncommon,” they offered no consideration of the odds of mutations rearranging these two genes in a fashion that is functional and performs some new and useful function.
j. Dntf-2r
This study, co-authored by Long, claimed that Dntf-2r, a fruit fly gene, arose as a duplicate that was retrotransposed from the gene Dntf-2. Using Gene Evolution Game Rule 2, the authors attempt to explain the subsequent evolution of Dntf-2r by assessing the ratio of non-synonymous to synonymous differences. Using one test, they found that “polymorphism is higher for synonymous than for replacement sites … revealing the action of purifying selection,” however another test “revealed a significant excess of amino acid substitutions, suggesting that the accelerated protein sequence evolution is likely a consequence of the action of positive Darwinian selection.” To explain these seemingly contrary results, they decided that “both purifying selection and adaptive evolution” were at work. But they did not try to explain exactly what functions these forces were working to preserve or to change because the authors didn’t know the function of Dntf-2r. Before their study “there was no information on the function of Dntf-2r” and after their study, all they could say was “this gene may produce a functional protein.” Once again, positive selection is being conjured even though it is “divorced from any biological mechanism.”136 One would certainly like to know the mutational pathway taken or the selective advantage offered by specific mutations along that pathway. None of this is discussed, meaning an explanation for the evolution of new genetic information is absent from this paper.
The authors also tried to explain the origin of the promoter for Dntf-2r, rightly noting that “Whether or not a retroposed sequence recruits a new promoter is a critical step to its future fate. If a retroposed sequence integrates in a genomic region devoid of expression potential, it would be doomed to evolve into a pseudogene.” So how did Dntf-2r get its promoter? The authors found that Dntf-2r‘s promoter fortuitously comes from DNA near where it’s located (its insertion site), but state that “it is unclear if this previously existing sequence is a functional promoter for some unknown gene in the region or is just a random genomic sequence that happens to be similar to a promoter sequence.”137 The authors make no attempt to assess the plausibility of these alternatives: they assess neither the likelihood of a “random genomic sequence” suddenly becoming a functional promoter sequence, nor the likelihood of a gene being inserted by chance right next to a functional promoter.
k. Sanguinaria rps1
This paper was inspired by the finding of “three striking distributional anomalies in a survey of mitochondrial gene content in angiosperms.”138 In other words, they found genes in species where they weren’t expected under the conventional understanding of common descent, because the same genes were found in supposedly “distantly related flowering plants.”
Following Ragan and Beiko (“topological discordance between a gene tree and a trusted reference tree is taken as a prima facie instance of LGT [lateral gene transfer]”)139, the authors assume that this phylogenetic incongruity is the result of LGT. This paper thus did not really explain the actual origin of these genes, but simply assumed and asserted that wherever and however they evolved, the genes were transplanted into these flowering plants via LGT (also known as horizontal gene transfer, or “HGT”).
The authors conclude that these data “establish for the first time that conventional genes are subject to evolutionarily frequent HGT during plant evolution and provide the first unambiguous evidence that plants can donate DNA horizontally to other plants.” Yet the authors admitted that the question “How do genes move from one plant to another, sexually unrelated, plant?” remains unanswered. Thus, evidence for HGT in plants is based merely upon the incongruent distribution of these genes assuming the standard phylogeny, not any actually established mechanism of HGT in flowering plants. Indeed, the authors admit that “horizontal transfer is unknown within the evolution of animals, plants and fungi except in the special context of mobile genetic elements.” This paper thus tells us virtually nothing about the actual original evolutionary birth of these genes, wherever they first originated, and instead highlights the assumptions and ad hoc reasoning used to save common descent from falsification by contrary phylogenetic data.
While studying this gene in various plant species, the authors found two additional instances of HGT, one of which was in Sanguinaria canadensis (bloodroot), a dicot whose rps11 gene “turns out to be chimaeric: its 5′ half is of expected eudicot, vertical origin, but its 3′ half is indisputably of monocot, horizontal origin.” In other words, half the gene appears like dicot rps11 and the other half appears like monocot rps11, and it is therefore identified as “chimaeric.” According to this story, monocot rps11 was transported into the Sanguinaria genome (by an unknown mechanism) and then, just by chance, happened to fuse with the dicot version of the same gene to create a new functional gene. The authors never discuss whether it is remotely plausible to claim that a gene would be transported from another species (by an unknown mechanism) only to fuse with its own homologue in the new genome—just by chance—and then create a new functional gene.
l. PMCHL
Despite the NCSE’s smooth assurance that “Biologists have no trouble showing how new information (in the sense used by information theorists) originates, nor how new genes, kinds of cells or tissues evolve,” this 2001 paper opens by admitting that “How genes with newly characterized functions originate remains a fundamental question.”140 Like the Sdic and AFGP examples, the origin of PMCHL1 and PMCHL2, considered here, required the “de novo” creation of key components of the gene where an exon “originated from a unique noncoding sequence.”
The authors describe this process as requiring the “creation of 3′ exons from a unique noncoding genomic sequence that fortuitously evolved as a standard intron-exon structure and polyadenylation signal sequences.” Key portions of this gene therefore just “fortuitously evolved.” Is that an explanation? The paper does not want to encourage such arbitrary explanations, and thus the authors caution that “de novo generation of building blocks—single genes or gene segments coding for protein domains— seems to be rare.”
Accounting for the origin of the rest of this gene proved extremely complicated, but Gene Evolution Game Rules 1 and 3 allowed the authors to invoke a series of rearrangements including retrotranspositions, insertions, and duplications. They propose that these genes were suddenly “co-opted” or “‘exapted’ into a functional role.” While the origin of genes with new functions is indeed a “fundamental question,” this paper’s reliance on “fortuitously evolved” explanations does very little to answer that question. This is especially true considering that the authors offered no analysis of the mutational odds of converting noncoding DNA to coding DNA and recruiting and rearranging multiple segments of the genome to create a new functional gene.
8. What does Long et al. really tell us?
Additional, similar examples from Long et al. could be given, but the point is clear enough already: a careful analysis of Long et al. exposes the utterly insufficient explanations offered by neo-Darwinists to account for the origin of new genetic information.
In not a single case did the above papers cited by Long et al. actually explain how new functional information arose. In other words, in no case was there an analysis of how natural selection could have favored mutational changes that were shown to be likely along each step of an alleged evolutionary pathway; never was any detailed step-by-step mutational pathway even given. At best, these studies offered vague and ad hoc appeals to duplication, rearrangement, and natural selection—often in a sudden, extreme, and abrupt manner—to form the gene in question. In many cases, natural selection was invoked to allegedly account for changes in the gene, even though the investigators didn’t even know the function of the gene and thereby could not identify the advantage provided by the gene’s function. In no case were calculations performed to assess whether sufficient probabilistic resources existed to produce the asserted mutational events on a reasonable timescale. In some cases, the original genetic material for the genes was unknown, or the studies asserted spontaneous “de novo” origin of genes from previously non-coding DNA. While they readily admitted that “de novo” gene emergence is rare, no attempt was made to assess whether such an unguided mechanism is even remotely plausible on mathematical probabilistic grounds. These papers play the Gene Evolution Game, but never explain how neo-Darwinian mechanisms create new genetic information.
By citing Long et al., the NCSE is basically engaging in a tactic known as citation-bluffing.
Properly understood, papers like Long et al. highlight the utter vagueness of Darwinian explanations for the origin of new genes. Thus, the NCSE states:
As it has become more practical to trace the sequences of genes in multiple species, scientists have been able to identify genes which went through these processes, acquiring new functions within relatively recent history. That research systematically refutes the claim in Explore Evolution that “whether you’re talking about artificial selection or about microevolution that occurs naturally, changes in the sub-population take place as genetic information is lost to that population” (p. 95).
Yet as discussed, the NCSE’s citation bluffs have not really answered any of the questions posed by EE. Instead, the mechanisms invoked in these papers are vague and hypothetical at best:
- exons may have been “recruited” or “donated” from other genes (and in some cases from an “unknown sou[r]ce”);
- there were vague appeals to “extensive refashioning of the genome”;
- mutations were said to cause “fortuitous juxtaposition of suitable sequences” in a gene-promoting region that therefore “did not really ‘evolve'”;
- researchers assumed “radical change in the structure” due to “rapid, adaptive evolution” and claimed that “positive selection has played an important role in the evolution” of the gene, even though function of the gene was not even known;
- genes were purportedly “cobbled together from DNA of no related function (or no function at all)”;
- the “creation” of new exons “from a unique noncoding genomic sequence that fortuitously evolved” was assumed, not demonstrated;
- we were given alternatives that promoter regions arose from a “random genomic sequence that happens to be similar to a promoter sequence,” or that the gene arose because it was inserted by pure chance right next to a functional promoter.
- explanations went little further than invoking “the chimeric fusion of two genes” based solely on sequence similarity;
- when no source material is recognizable, we’re told that “genes emerge and evolve very rapidly, generating copies that bear little similarity to their ancestral precursors” because they are simply “hypermutable”;
- we even saw “a striking case of convergent evolution” of “near-identical” proteins.
To reiterate, in no cases were the odds of these unlikely events taking place actually calculated.
Incredibly, natural selection was repeatedly invoked in instances where the investigators did not know the function of the gene being studied and thus could not possibly have identified any known functional advantages gained through the mutations being invoked. In the case where multiple mutational steps were involved, no tests were done of the functional viability of the alleged intermediate stages. These papers offer vague stories but not viable, plausibly demonstrated explanations for the origin of new genetic information.
Within modern evolutionary biology, there are indeed many unanswered questions about how unguided selection acting upon random mutation produces new functional biological information. But the NCSE pretends that fundamental scientific questions have been answered, when no adequate answers exist, at least not from the neo-Darwinian paradigm.
As a result, the NCSE and not only miseducates students, it threatens to hinder scientific progress by pretending that some of the most important questions in biology are answered, when they really aren’t. The myth unfortunately has become a pillar of how evolution is explained and defended in academia, education and the media. EE is eminently justified in explaining why critics harbor doubts about the ability of neo-Darwinian evolution to account for the origin of new genetic information.
VI. Losing Information
While neo-Darwinian evolution is not good at creating new genetic information, it is very good at destroying genetic information. As noted above, the NCSE criticizes EE for observing that most evolution takes place in processes that mandate the loss of genetic information.
Yet as we have seen, the NCSE has not demonstrated that neo-Darwinian processes are capable of producing new genetic information. Moreover, the NCSE is taking EE dramatically out of context. The full quote from EE is as follows:
On the other hand, each daughter population will have lost genetic information necessary for building certain other traits. The total biological information in the gene pool will have decreased, which limits how much the daughter population can vary and change in the future. Ultimately, this means that the isolated “daughter” populations are more vulnerable to environmental stresses (natural disasters or other changes in the environment). For this reason, small isolated populations are great candidates for extinction. In summary, whether you’re talking about artificial selection or about microevolution that occurs naturally, changes in the sub-population take place as genetic information is lost to that population. (pg. 95)
EE’s point is eminently reasonable, for it’s difficult to deny that the processes behind speciation necessarily entail a reduction in the gene pool. Basic evolutionary biology postulates that speciation takes place in reproductively isolated populations that are typically small. The isolated population therefore has less genetic diversity—not more—than the parent population.
Thus, reproductive isolation—the mechanism that is supposed to foster evolutionary change—arises during a process requiring a reduction in the size of the gene pool. Darwin understood this problem, as he wrote in the Origin of Species that “forms existing in larger numbers will always have a better chance, within any given period, of presenting further favorable variations for natural selection to seize on, than will the rarer forms which exist in lesser numbers.”141 Mechanisms of evolution that reduce the size of the population, logically speaking, will present fewer opportunities for “favorable variations for natural selection to seize on.”
Additionally, Michael Lynch observes that generally speaking, “Multicellular species experience reduced population sizes, reduced recombination rates, and increased deleterious mutation rates, all of which diminish the efficiency of selection.”142 Small populations tend to be inbred, and inbreeding tends to reduce genetic variation,143 thus leading to the problem of inbreeding depression, where deleterious alleles become common.
Small populations decrease the amount of genetic information and provide fewer opportunities for new genetic information to arise. EE’s point is not refuted.
Conclusion
The NCSE claims that EE has been “systematically refuted” regarding the origin of new genetic information, but a closer look at the NCSE’s citations exposes citations bluffs that do not support the NCSE’s claim that “Biologists have no trouble showing how new information (in the sense used by information theorists) originates, nor how new genes, kinds of cells or tissues evolve.”
The NCSE boasts that neo-Darwinian evolutionary mechanisms produce new genetic information, but scrutinizing the NCSE’s sources shows no such thing. Rather than trying to stifle investigation into questions about the evolutionary origin of new genetic information by appeals to vague and insufficient explanations, the NCSE ought to follow the approach of EE and ask students to consider points like this:
These critics would say that natural selection works well as an editor, but not an author. It has a demonstrated capacity to weed out the failures from among what already exists, but it has not been shown to generate new biological information or structures. (EE, pg. 95)
Such honest assessments of the data will inspire students to adequately investigate neo-Darwinian mechanisms. Perhaps this could even inspire students to ultimately solve these problems and explain how neo-Darwinism can explain the origin of new genetic information. Or perhaps they will inspire students to do research that leads to the abandonment of neo-Darwinism as a paradigm. Perhaps for the NCSE the risk of the latter outcome is too great to encourage students in research that could, alternatively, lead to the former outcome.
Regardless, it seems clear that EE is asking students to investigate fundamental and important questions about neo-Darwinian evolution. These are cutting-edge questions that are being addressed by mainstream biologists. Given the weak, vague, and wholly inadequate explanations offered by authorities cited by the NCSE, EE appears justified in concluding that natural selection “has not been shown to generate new biological information or structures.”
References Cited:
[1.] Of course neo-Darwinian evolution asserts that other processes, such as random genetic drift, are at work, but natural selection is still said to be the primary force driving the generatation of the adaptive complexity of life. Drift is not an adaptive mechanism, and the insufficiency of drift to explain biological complexity will also be discussed further in this response to the NCSE.
[2.] John A. Endler, Natural Selection in the Wild, pg. 247 (Princeton University Press, 1986).
[3.] John A. Endler, Natural Selection in the Wild, pgs. 248-249 (Princeton University Press, 1986).
[4.] Michael A. Bell, “Gould’s most cherished concept, review of Punctuated Equilibrium by Stephen Jay Gould. Belknap Press of Harvard University Press, 2007,” Trends in Ecology and Evolution, Vol. 23(3):121-122 (2008) (emphasis added).
[5.] John Whitfield, “Biological Theory: Postmodern evolution?,” Nature, Vol. 455:281-284 (2008).
[6.] Stewart Newman quoted in John Whitfield, “Biological Theory: Postmodern evolution?,” Nature, Vol. 455:281-284 (2008).
[7.] Graham Budd quoted in John Whitfield, “Biological Theory: Postmodern evolution?,” Nature, Vol. 455:281-284 (2008).
[8.] William Provine, Random Drift and the Evolutionary Synthesis, History of Science Society HSS Abstracts
[9.] Eugene V. Koonin, “The Origin at 150: Is a New Evolutionary Synthesis in Sight?,” Trends in Genetics, Vol. 473:474 (2009) (internal citations omitted).
[10.] Id.
[11.] Günter Theißen, “Saltational evolution: hopeful monsters are here to stay,” Theory in Biosciences, Vol. 128:43-51 (2009).
[12.] Günter Theißen, “The proper place of hopeful monsters in evolutionary biology,” Theory in Biosciences, Vol. 124:349-369 (2006).
[13.] Id.
[14.] Michael Lynch, “The frailty of adaptive hypotheses for the origins of organismal complexity,” Proceedings of the National Academy of Sciences, Vol. 104:8597-8604 (May 15, 2007).
[15.] Id.
[16.] Loeske E. B. Kruuk, “Estimating genetic parameters in natural populations using the ‘animal model’,” Philosophical Transactions of the Royal Society of London B, Vol. 359:873-890 (2004) (internal citations removed).
[17.] Montgomery Slatkin and Mark Kirkpatrick, “Extrapolating Quantitative Genetic Theory to Evolutionary Problems,” in Evolutionary Genetics of Invertebrate Behavior: Progress and Prospects, pg. 291 (ed. Milton Davis Huettel, New York: Plenum, 1986).
[18.] Mary Jane West-Eberhard, Developmental Plasticity and Evolution, pg. 155 (Oxford University Press US, 2003).
[19.] The NCSE states: “Explore Evolution argues that if natural selection cannot produce a certain change in a matter of decades, it could never produce that change. This is nonsensical on its face, and does not accurately reflect basic knowledge about natural selection and population genetics stretching back to the 1920s.”
[20.] “This does not detract from the significance of artificial selection as an assay for genetic variation in natural populations. [Artificial selection] means the potential for a response to selection. Though evolution may not be as rapid in natural populations, the time spans for selection to operate are much longer.” Mary Jane West-Eberhard, Developmental Plasticity and Evolution, pgs. 155-156 (Oxford University Press US, 2003).
[21.] https://dictionary.reference.com/browse/analogy
[22.] George John Romains, Darwin, and After Darwin: An Exposition of the Darwinian Theory and a Discussion of Post-Darwinian Questions Volume 1, pg. 296 (Open Court Publishing Company, 1910) (emphasis added).
[23.] George St. Clair, Darwinism and Design; or Creation by Evolution, pg. 108 (Hodder and Stoughton, 1873) (emphasis added).
[24.] Charles Clement Coe, Nature versus natural selection: an essay on organic evolution, pg. 130 (Swan Sonnenschein & Co, 1895) (emphasis added).
[25.] Largent, Mark A. “Darwin’s Analogy between Artificial and Natural Selection in the Origin of Species,” in The Cambridge Companion to the “Origin of Species”. (Michael Ruse and Robert J. Richards, Eds. Cambridge University Press, 2008) (emphasis added).
[26.] C. Kenneth Waters, “The Arguments in Origin of Species,” in The Cambridge Companion to Darwin, pg. 120 (Jonathan Hodge & Gregory Radick eds., Cambridge University Press, 2003) (emphasis added).
[27.] Jeffrey Ross-Ibarra, Peter L. Morrell, and Brandon S. Gaut, “Plant Domestication, a Unique Opportunity to Identify the Genetic Basis of Adaptation,” in In the Light of Evolution, Volume 1: Adaptation and Complex Design, pg. 208 (John C. Avise, Francisco José Ayala eds., National Academies Press, 2007) (originally published in Proceedings of the National Academy of Sciences, Vol. 104:8641-8648 May 15, 2007) (emphasis added).
[28.] M. S. J. Hodge, “Natural Selection: Historical Perspectives,” in Keywords in Evolutionary Biology, pgs. 212-213 (Evelyn Fox Keller and Elisabeth Anne Lloyd eds., Harvard University Press, 1992) (emphasis added).
[29.] James A. Shapiro, “Genome system architecture and natural genetic engineering in evolution,” Annals of the New York Academy of Sciences, Vol. 870:23-35 (May 18, 1999) (emphasis added).
[30.] Matt Ridley, Evolution, pg. 261 (3rd Ed., Blackwell, 2004).
[31.] Id.
[32.] Sean B. Carroll, The Making of the Fittest: DNA and the Ultimate Forensic Record of Evolution, pg. 197 (W. W. Norton, 2006).
[33.] Michael J. Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, pg. 38 (Free Press, 1996).
[34.] For example, see abrupt appearance of these features in diagrams in the following textbooks, all of which use essentially the same diagram to explain the origin of the eye: Matt Ridley, Evolution, pg. 261, figure 10.2, steps (e) and (f) (3rd Ed., Blackwell, 2004); Brian K. Hall, Benedikt Hallgrimsson, Strickberger’s Evolution: The Integration of Genes, Organisms, and Populations, pg. 38, Box 3-1, steps (e) and (f) (4th ed., Jones and Bartlett, 2008); Scott Freeman & Jon C. Herron, Evolutionary Analysis, pg. 98, step(d) (3rd Ed., Prentice Hall, 2004).
[35.] John Whitfield, “Biological Theory: Postmodern evolution?,” Nature, Vol. 455:281-284 (2008).
[36.] Francisco J. Ayala, Darwin’s Gift to Science and Religion, pg. 146 (Joseph Henry Press, 2007).
[37.] Michael J. Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, pg. 15 (Free Press, 1996).
[38.] David Berlinski, “Keeping an Eye on Evolution: Richard Dawkins, a relentless Darwinian spear carrier, trips over Mount Improbable. Review of Climbing Mount Improbable by Richard Dawkins (W. H. Norton & Company, Inc. 1996),” in The Globe & Mail (November 2, 1996).
[39.] For example, the following textbooks surveyed did not discuss HIV evolution: Kenneth Miller and Joseph Levine, Biology (Pearson/Prentice Hall, 2008); Sylva S. Mader’s Essentials of Biology (McGraw Hill, 2007); Strauss and Lisowski’s Biology: The Web of Life (Addison-Wesley, 2000); Joseph Raver, Biology: Patterns and Processes of Life (J. M. Lebel, 2004); Glencoe Biology: An Everyday Experience (Glencoe, 2003).
[40.] The following textbooks did not discuss insect resistance to insecticides: Kenneth Miller and Joseph Levine, Biology (Pearson/Prentice Hall, 2008); Sylva S. Mader’s Essentials of Biology (McGraw Hill, 2007); Strauss and Lisowski’s Biology: The Web of Life (Addison-Wesley, 2000); Joseph Raver, Biology: Patterns and Processes of Life (J. M. Lebel, 2004); Collen Belk & Virginia Borden Maier, Biology: Science for Life (Pearson / Benjamin Cummings, 2010); Sylvia S. Mader, Biology (10th Ed., McGraw Hill, 2007); Glencoe Biology: An Everyday Experience (Glencoe, 2003).
[41.] For example the following textbooks surveyed make no mention of latitudinal gradients in allele frequency in fruit fly populations, guppy coloration changes in response to local predation pressures, and paleontological evidence of snail shell shapes showing stabilizing selection: Kenneth Miller and Joseph Levine, Biology (Pearson/Prentice Hall, 2008); Strauss and Lisowski’s Biology: The Web of Life (Addison-Wesley, 2000); Glencoe’s Biology: The Dynamics of Life (Florida Edition, 2006); Joseph Raver Biology: Patterns and Processes of Life (J. M. Lebel, 2004); Collen Belk & Virginia Borden Maier, Biology: Science for Life (Pearson / Benjamin Cummings, 2010); Sylvia S. Mader, Biology (10th Ed., McGraw Hill, 2007); Glencoe Biology: An Everyday Experience (Glencoe, 2003); Scott Freeman, Biological Science (3rd. Ed., 2008).
[42.] See K. Kris Hirst, “Animal Domestication: Table of Dates and Places.”
[43.] See Teaching About Evolution and the Nature of Science (National Academy Press, 1998), or Niles Eldredge, The Triumph of Evolution and the Failure of Creationism (2000).
[44.] B. Gaffney and E.P. Cunningham, “Estimation of genetic trend in racing performance of thoroughbred horses,” Nature, Vol. 332:722-724 (April, 21, 1988).
[45.] Ernest Bailey, “Odds on the FAST Gene,” Genome Research, Vol. 8:569-571 (1998).
[46.] Ernest Bailey, “Odds on the FAST Gene,” Genome Research, Vol. 8:569-571 (1998) (emphasis added).
[47.] Id.
[48.] Id.
[49.] See Sarah Mace, “NYTB ‘Breeder Spotlight’ Paul H. Rothfuss,” Director’s Corner Blog of New York Thoroughbred Breeders, Inc. (June 10, 2009).
[50.] Paul H. Rothfuss, “Race Horses — ‘Softer’ Than Before? — Article III.”
[51.] Private correspondence with Paul H. Rothfuss on August 26, 2009 (emphases added).
[52.] Austin L. Hughes, “Looking for Darwin in all the wrong places: the misguided quest for positive selection at the nucleotide sequence level,” Heredity, Vol. 99:364-373 (2007).
[53.] Ernst Mayr, What Evolution Is, pg. 140 (Basic Books, 2001).
[54.] Id.
[55.] Id.
[56.] Sean B. Carroll, “The Big Picture,” Nature, Vol. 409:669 (Feb. 8, 2001).
[57.] Matt Ridley, Evolution, pg. 552 (3rd Ed., Blackwell, 2004).
[58.] David Sepkoski, “Macroevolution,” in The Oxford Handbook of Philosophy of Biology, pgs. 211-212 (Michael Ruse, Ed., Oxford University Press, 2008).
[59.] Joseph Travis and David N. Resnick, “Adaptation,” in Evolution: The First Four Billion Years, pg. 126 (Michael Ruse, Edward O. Wilson, Joseph Travis eds., Harvard University Press, 2009).
[60.] Peter H. Raven & George B. Johnson, Biology, pg. 455 (6th ed. 2002).
[61.] Neil A. Campbell & Jane B. Reece, Biology, pg. 486 (6th ed., 2002).
[62.] Kevin J. Peterson, Michael R. Dietrich and Mark A. McPeek, “MicroRNAs and metazoan macroevolution: insights into canalization, complexity, and the Cambrian explosion,” BioEssays, Vol. 31 (7):736 – 747 (2009).
[63.] Nicholas Matzke quoted in Michael Powell, “Controversial Editor Backed,” Washington Post (August 19, 2005).
[64.] Kenneth R. Miller, Kitzmiller v. Dover Day 1 AM testimony, pg. 135 (September 26, 2005).
[65.] Kitzmiller v. Dover, 400 F.Supp.2d 707, 744 (M.D.Pa. 2005).
[66.] Manyuan Long, Esther Betrán, Kevin Thornton, and Wen Wang, “The Origin of New Genes: Glimpses from the Young and Old,” Nature Reviews Genetics, Vol. 4:865-875 (November, 2003).
[67.] The word “information” appears once in the entire article—in the title of note 103. Id. at 875 n. 103. See Manyuan Long, Esther Betrán, Kevin Thornton, and Wen Wang, “The Origin of New Genes: Glimpses from the Young and Old,” Nature Reviews Genetics, Vol. 4:865-875 (November, 2003).
[68.] Nicholas J. Matzke and Paul R. Gross, “Analyzing Critical Analysis: The Fallback Antievolutionist Strategy,” pg. 42 in Not in Our Classrooms: Why Intelligent Design is Wrong for Our Schools (edited by Eugenie C. Scott and Glenn Branch, Beacon Press, 2006).
[69.] Id.
[70.] This calculation uses a 26-letter English alphabet that is not case-sensitive and, as seen in the strings, does not use spaces.
[71.] String B was generated using a random character generator from the website Random.org.
[72.] Jack W. Szostak, “Molecular messages,” Nature, Vol. 423:689 (June 12, 2003).
[73.] Kirk K. Durston, David K. Y. Chiu, David L. Abel, Jack T. Trevors, “Measuring the functional sequence complexity of proteins,” Theoretical Biology and Medical Modelling, Vol. 4:47 (2007) (internal citations removed).
[74.] Robert M. Hazen, Patrick L. Griffin, James M. Carothers, and Jack W. Szostak, “Functional information and the emergence of biocomplexity,” Proceedings of the National Academy of Sciences, USA, Vol. 104:8574-8581 (May 15, 2007).
[75.] Stephen C. Meyer, “The origin of biological information and the higher taxonomic categories,” Proceedings of the Biological Society of Washington, Vol. 117(2):213-239 (2004).
[76.] Leslie E. Orgel, The Origins of Life: Molecules and Natural Selection, pg. 189 (Chapman & Hall: London, 1973).
[77.] Hubert P. Yockey, “Self Organization Origin of Life Scenarios and Information Theory,” Journal of Theoretical Biology, Vol. 91:13-31 (1981).
[78.] Richard Sternberg, “DNA Codes and Information: Formal Structures and Relational Causes,” Acta Biotheoretica, Vol. 56(3):205-32 (September, 2008).
[79.] Id.
[80.] Jack T. Trevors and David L. Abel, “Chance and necessity do not explain the origin of life,” Cell Biology International, Vol. 28: 729-739 (2004).
[81.] Øyvind Albert Voie, “Biological function and the genetic code are interdependent,” Chaos, Solitons and Fractals, Vol. 28(4): 1000-1004 (2006).
[82.] Comment by Michael Egnor at http://scienceblogs.com/pharyngula/2007/02/dr_michael_egnor_challenges_ev.php#comment-349555 (February 20, 2007).
[83.] Again, as implied in the body, if one could predict the string would be duplicated, then the Shannon Information would also not increase after duplicating the string, in which case there is no increase in CSI nor Shannon Information.
[84.] Michael Lynch, “The frailty of adaptive hypotheses for the origins of organismal complexity,” Proceedings of the National Academy of Sciences, Vol. 104:8597-8604 (May 15, 2007).
[85.] Austin L. Hughes, “The origin of adaptive phenotypes,” Proceedings of the National Academy of Sciences USA, Vol. 105(36):13193-13194 (Sept. 9, 2008) (internal citations removed).
[86.] Michael J. Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, pgs. 175-176 (Free Press, 1996).
[87.] Michael J. Behe, The Edge of Evolution: The Search for the Limits of Darwinism, pg. 95 (Free Press, 2007).
[88.] See for example, “Limits on Evolution” at https://ncse.com/creationism/analysis/extrapolations.
[89.] For example, when the ratio of nonsynonymous (i.e. amino acid changing) to synonymous (i.e. non-amino acid changing) differences between Gene B and Gene A is high, we have can say that it must be natural selection at work because only strong selection pressure would preserve so many changes that change amino acid sequence. Incredibly, we can also say that when the same ratio is low (i.e. there are FEW amino acid replacements in a gene), that too shows that natural selection was at work, in this case in the form of stabilizing selection to conserve gene sequence. This approach was taken in Harmit S. Malik and Steven Henikoff, “Adaptive Evolution of Cid, a Centromere-Specific Histone in Drosophila,” Genetics, Vol. 157:1293-1298 (March 2001) and its discussion of the Cid gene in the text.
[90.] See for example Ulfar Bergthorsson, Keith L. Adams, Brendan Thomason, and Jeffrey D. Palmer, “Widespread horizontal transfer of mitochondrial genes in flowering plants,” Nature, Vol. 424:197-201 (July 10, 2003). See also Mark A. Ragan and Robert G. Beiko, “Lateral genetic transfer: open issues,” Philosophical Transactions of the Royal Society B, Vol. 364:2241-2251 (2009) (“topological discordance between a gene tree and a trusted reference tree is taken as a prima facie instance of LGT”).
[91.] For example, this explanation was invoked in Matthew E. Johnson, Luigi Viggiano, Jeffrey A. Bailey, Munah Abdul-Rauf, Graham Goodwin, Mariano Rocchi & Evan E. Eichler, “Positive selection of a gene family during the emergence of humans and African apes,” Nature, Vol. 413:514-519 (October 4, 2001).
[92.] Austin L. Hughes, “Looking for Darwin in all the wrong places: the misguided quest for positive selection at the nucleotide sequence level,” Heredity, Vol. 99:364-373 (2007).
[93.] Id.
[94.] Id.
[95.] “The modern synthesis is good at modeling the survival of the fittest, but not the arrival of the fittest.” Scott Gilbert, quoted in John Whitfield, “Biological Theory: Postmodern evolution?,” Nature, Vol. 455:281-284 (2008).
[96.] Bernard Wood, quoted in Joseph B. Verrengia, “Gene Mutation Said Linked to Evolution,” Associated Press, found in San Diego Union Tribune, March 24, 2004.
[97.] Jerry Coyne, “The Great Mutator,” The New Republic (June 14, 2007). Coyne asserts he knows of no example where this is the case.
[98.] David Berlinski, “Keeping an Eye on Evolution: Richard Dawkins, a relentless Darwinian spear carrier, trips over Mount Improbable. Review of Climbing Mount Improbable by Richard Dawkins (W. H. Norton & Company, Inc. 1996),” in The Globe & Mail (November 2, 1996).
[99.] Douglas A. Axe, “Estimating the Prevalence of Protein Sequences Adopting Functional Enzyme Folds,” Journal of Molecular Biology, Vol. 341: 1295-1315 (2004); Douglas A. Axe, “Extreme Functional Sensitivity to Conservative Amino Acid Changes on Enzyme Exteriors,” Journal of Molecular Biology, Vol. 301: 585-595 (2000).
[100.] See Stephen C. Meyer, Signature in the Cell: DNA and the Evidence for Intelligent Design, pg. 211 (HarperOne, 2009).
[101.] Neil A. Campbell and Jane B. Reece, Biology, pg. 84 (7th ed, 2005).
[102.] David S. Goodsell, The Machinery of Life, pg. 17, 19 (2nd ed, Springer, 2009).
[103.] Michael J. Behe & David W. Snoke, “Simulating Evolution by Gene Duplication of Protein Features That Require Multiple Amino Acid Residues,” Protein Science, Vol 13:2651-2664 (2004).
[104.] Rick Durrett and Deena Schmidt, “Waiting for Two Mutations: With Applications to Regulatory Sequence Evolution and the Limits of Darwinian Evolution,” Genetics, Vol. 180: 1501-1509 (November 2008).
[105.] Michael Behe, The Edge of Evolution: The Search for the Limits of Darwinism, Appendix D, pgs. 272-275 (Free Press, 2007) (emphasis added).
[106.] Austin L. Hughes, “The origin of adaptive phenotypes,” Proceedings of the National Academy of Sciences USA, Vol. 105(36):13193-13194 (Sept. 9, 2008) (internal citations removed).
[107.] Michael Lynch, “The frailty of adaptive hypotheses for the origins of organismal complexity,” Proceedings of the National Academy of Sciences, Vol. 104:8597-8604 (May 15, 2007).
[108.] Manyuan Long, Esther Betrán, Kevin Thornton, and Wen Wang, “The Origin of New Genes: Glimpses from the Young and Old,” Nature Reviews Genetics, Vol. 4:865-875 (November, 2003).
[109.] See Limits on Evolution at https://ncse.com/creationism/analysis/extrapolations
[110.] Manyuan Long & Charles H. Langley, “Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila,” Science, Vol. 260:91-95 (April 2, 1993).
[111.] John M. Logsdon, Jr., & W. Ford Doolittle, “Origin of antifreeze protein genes: A cool tale in molecular evolution,” Proceedings of the National Academy of Sciences USA, Vol. 94:3485-3487 (April, 1997).
[112.] Austin L. Hughes, “Looking for Darwin in all the wrong places: the misguided quest for positive selection at the nucleotide sequence level,” Heredity, Vol. 99:364-373 (2007).
[113.] Dmitry I. Nurminsky, Maria V. Nurminskaya, Daniel De Aguiar, and Daniel L. Hartl, “Selective sweep of a newly evolved sperm-specic gene in Drosophila,” Nature, Vol. 396:572-575 (December 10, 1998).
[114.] Id.
[115.] Manyuan Long, Esther Betrán, Kevin Thornton, and Wen Wang, “The Origin of New Genes: Glimpses from the Young and Old,” Nature Reviews Genetics, Vol. 4:865-875 (November, 2003).
[116.] Harmit S. Malik and Steven Henikoff, “Adaptive Evolution of Cid, a Centromere-Specific Histone in Drosophila,” Genetics, Vol. 157:1293-1298 (March 2001).
[117.] Austin L. Hughes, “The origin of adaptive phenotypes,” Proceedings of the National Academy of Sciences USA, Vol. 105(36):13193-13194 (Sept. 9, 2008) (internal citations removed).
[118.] Liangbiao Chen, Arthur L. DeVries, & Chi-Hing C. Cheng, “Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod,” Proceedings of the National Academy of Sciences USA, Vol. 94:3817-3822 (April, 1997).
[119.] John M. Logsdon, Jr., & W. Ford Doolittle, “Origin of antifreeze protein genes: A cool tale in molecular evolution,” Proceedings of the National Academy of Sciences USA, Vol. 94:3485-3487 (April, 1997).
[120.] John M. Logsdon, Jr., & W. Ford Doolittle, “Origin of antifreeze protein genes: A cool tale in molecular evolution,” Proceedings of the National Academy of Sciences USA, Vol. 94:3485-3487 (April, 1997).
[121.] John M. Logsdon, Jr., & W. Ford Doolittle, “Origin of antifreeze protein genes: A cool tale in molecular evolution,” Proceedings of the National Academy of Sciences USA, Vol. 94:3485-3487 (April, 1997).
[122.] Liangbiao Chen, Arthur L. DeVries, & Chi-Hing C. Cheng, “Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish,” Proceedings of the National Academy of Sciences USA, Vol. 94:3811-3816 (April, 1997).
[123.] John M. Logsdon, Jr., & W. Ford Doolittle, “Origin of antifreeze protein genes: A cool tale in molecular evolution,” Proceedings of the National Academy of Sciences USA, Vol. 94:3485-3487 (April, 1997).
[124.] Liangbiao Chen, Arthur L. DeVries, & Chi-Hing C. Cheng, “Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish,” Proceedings of the National Academy of Sciences USA, Vol. 94:3811-3816 (April, 1997).
[125.] John M. Logsdon, Jr., & W. Ford Doolittle, “Origin of antifreeze protein genes: A cool tale in molecular evolution,” Proceedings of the National Academy of Sciences USA, Vol. 94:3485-3487 (April, 1997).
[126.] David J. Begun, “Origin and Evolution of a New Gene Descended From alcohol dehydrogenase in Drosophila,” Genetics, Vol. 145:375-382 (February, 1997).
[127.] Wolfgang Enard, Molly Przeworski, Simon E. Fisher, Cecilia S. L. Lai, Victor Wiebe, Takashi Kitano, Anthony P. Monaco & Svante Pääbo, “Molecular evolution of FOXP2, a gene involved in speech and language,” Nature, Vol. 418:869-872 (August 22, 2002) (stating “to establish whether FOXP2 is indeed involved in basic aspects of human culture, the normal functions of both the human and the chimpanzee FOXP2 proteins need to be clarified”).
[128.] Jianzhi Zhang, David M. Webb and Ondrej Podlaha, “Accelerated Protein Evolution and Origins of Human-Specific Features: FOXP2 as an Example,” Genetics, Vol. 162:1825-1835 (December 2002).
[129.] Id.
[130.] Id.
[131.] Austin L. Hughes, “Looking for Darwin in all the wrong places: the misguided quest for positive selection at the nucleotide sequence level,” Heredity, Vol. 99:364-373 (2007).
[132.] Manyuan Long, Sandro J. de Souza, Carl Rosenberg, and Walter Gilbert, “Exon shuffling and the origin of the mitochondrial targeting function in plant cytochrome cl precursor,” Proceedings of the National Academy of Sciences USA, Vol. 93:7727-7731 (July, 1996).
[133.] Id. Specifically, the authors write: “In a computer survey of an exon database, we observed a high similarity (44% identity and 64% similarity over 41 amino acids) between the 5′ three consecutive exons of the pea Gapc1 and the potato cytochrome c1 precursor.”
[134.] Matthew E. Johnson, Luigi Viggiano, Jeffrey A. Bailey, Munah Abdul-Rauf, Graham Goodwin, Mariano Rocchi & Evan E. Eichler, “Positive selection of a gene family during the emergence of humans and African apes,” Nature, Vol. 413:514-519 (October 4, 2001).
[135.] Charles A. Paulding, Maryellen Ruvolo, and Daniel A. Haber, “The Tre2 (USP6) oncogene is a hominoid-specific gene,” Proceedings of the National Academy of Sciences USA, Vol. 100(5):2507-2511 (March 4, 2003).
[136.] Austin L. Hughes, “Looking for Darwin in all the wrong places: the misguided quest for positive selection at the nucleotide sequence level,” Heredity, Vol. 99:364-373 (2007).
[137.] Esther Betran and Manyuan Long, “Dntf-2r, a Young Drosophila Retroposed Gene With Specific Male Expression Under Positive Darwinian Selection,” Genetics, Vol. 164:977-988 ( July 2003).
[138.] Ulfar Bergthorsson, Keith L. Adams, Brendan Thomason, and Jeffrey D. Palmer, “Widespread horizontal transfer of mitochondrial genes in flowering plants,” Nature, Vol. 424:197-201 (July 10, 2003).
[139.] Mark A. Ragan and Robert G. Beiko, “Lateral genetic transfer: open issues,” Philosophical Transactions of the Royal Society B, Vol. 364:2241-2251 (2009).
[140.] Anouk Courseaux and Jean-Louis Nahon, “Birth of Two Chimeric Genes in the Hominidae Lineage,” Science, Vol. 291:1293-1297 (February 16, 2001).
[141.] Darwin, Charles, The Origin of Species, “Chapter 6, Difficulties on Theory.”
[142.] Michael Lynch, “The frailty of adaptive hypotheses for the origins of organismal complexity,” Proceedings of the National Academy of Sciences, Vol. 104:8597-8604 (May 15, 2007).
[143.] See Benedikt Hallgrimsson, “Variation,” in Keywords and concepts in evolutionary developmental biology, pg. 370 (Brian K. Hall, Wendy Olson eds. Harvard University Press, 2006).